Inhibition of bone morphogenetic protein 6 receptors ameliorates Sjögren's syndrome in mice
- PMID: 32076051
- PMCID: PMC7031521
- DOI: 10.1038/s41598-020-59443-z
Inhibition of bone morphogenetic protein 6 receptors ameliorates Sjögren's syndrome in mice
Abstract
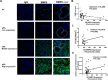
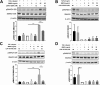
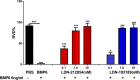
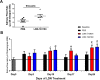
Primary Sjögren's syndrome (pSS) is a chronic autoimmune disease, with only palliative treatments available. Recent work has suggested that increased bone morphogenetic protein 6 (BMP6) expression could alter cell signaling in the salivary gland (SG) and result in the associated salivary hypofunction. We examined the prevalence of elevated BMP6 expression in a large cohort of pSS patients and tested the therapeutic efficacy of BMP signaling inhibitors in two pSS animal models. Increased BMP6 expression was found in the SGs of 54% of pSS patients, and this increased expression was correlated with low unstimulated whole saliva flow rate. In mouse models of SS, inhibition of BMP6 signaling reduced phosphorylation of SMAD1/5/8 in the mouse submandibular glands, and led to a recovery of SG function and a decrease in inflammatory markers in the mice. The recovery of SG function after inhibition of BMP6 signaling suggests cellular plasticity within the salivary gland and a possibility for therapeutic intervention that can reverse the loss of function in pSS.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Association of bone morphogenetic protein 6 with exocrine gland dysfunction in patients with Sjögren's syndrome and in mice.Arthritis Rheum. 2013 Dec;65(12):3228-38. doi: 10.1002/art.38123. Arthritis Rheum. 2013. PMID: 23982860 Free PMC article.
-
Restoration of CFTR Activity in Ducts Rescues Acinar Cell Function and Reduces Inflammation in Pancreatic and Salivary Glands of Mice.Gastroenterology. 2017 Oct;153(4):1148-1159. doi: 10.1053/j.gastro.2017.06.011. Epub 2017 Jun 19. Gastroenterology. 2017. PMID: 28634110 Free PMC article.
-
Progenitor cell niche senescence reflects pathology of the parotid salivary gland in primary Sjögren's syndrome.Rheumatology (Oxford). 2020 Oct 1;59(10):3003-3013. doi: 10.1093/rheumatology/keaa012. Rheumatology (Oxford). 2020. PMID: 32159757 Free PMC article.
-
Small-molecule inhibitors and the salivary gland epithelium in Sjögren's syndrome.Expert Opin Investig Drugs. 2019 Jul;28(7):605-616. doi: 10.1080/13543784.2019.1631796. Epub 2019 Jun 16. Expert Opin Investig Drugs. 2019. PMID: 31203680 Review.
-
The role of salivary gland histopathology in primary Sjögren's syndrome: promises and pitfalls.Clin Exp Rheumatol. 2018 May-Jun;36 Suppl 112(3):222-233. Epub 2018 Aug 14. Clin Exp Rheumatol. 2018. PMID: 30156550 Review.
Cited by
-
Transgenic dry eye mouse models: powerful tools to study dry eye disease.Int J Ophthalmol. 2022 Apr 18;15(4):635-645. doi: 10.18240/ijo.2022.04.18. eCollection 2022. Int J Ophthalmol. 2022. PMID: 35450189 Free PMC article. Review.
-
TGF-β Pathway in Salivary Gland Fibrosis.Int J Mol Sci. 2020 Nov 30;21(23):9138. doi: 10.3390/ijms21239138. Int J Mol Sci. 2020. PMID: 33266300 Free PMC article. Review.
-
Discovery of highly potent and ALK2/ALK1 selective kinase inhibitors using DNA-encoded chemistry technology.Proc Natl Acad Sci U S A. 2024 Nov 19;121(47):e2413108121. doi: 10.1073/pnas.2413108121. Epub 2024 Nov 14. Proc Natl Acad Sci U S A. 2024. PMID: 39541346 Free PMC article.
-
Lysosome-Associated Membrane Protein 3 Induces Lysosome-Dependent Cell Death by Impairing Autophagic Caspase 8 Degradation in the Salivary Glands of Individuals With Sjögren's Disease.Arthritis Rheumatol. 2023 Sep;75(9):1586-1598. doi: 10.1002/art.42540. Epub 2023 Jul 27. Arthritis Rheumatol. 2023. PMID: 37096570 Free PMC article.
-
Sclerosing Sialadenitis Is Associated With Salivary Gland Hypofunction and a Unique Gene Expression Profile in Sjögren's Syndrome.Front Immunol. 2021 Jul 30;12:699722. doi: 10.3389/fimmu.2021.699722. eCollection 2021. Front Immunol. 2021. PMID: 34400910 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

