A case of malignant hyperlactaemic acidosis appearing upon treatment with the mono-carboxylase transporter 1 inhibitor AZD3965
- PMID: 32076124
- PMCID: PMC7156442
- DOI: 10.1038/s41416-020-0727-8
A case of malignant hyperlactaemic acidosis appearing upon treatment with the mono-carboxylase transporter 1 inhibitor AZD3965
Erratum in
-
Correction: A case of malignant hyperlactaemic acidosis appearing upon treatment with the mono-carboxylase transporter 1 inhibitor AZD3965.Br J Cancer. 2020 Apr;122(8):1272. doi: 10.1038/s41416-020-0801-2. Br J Cancer. 2020. PMID: 32203218 Free PMC article.
Abstract
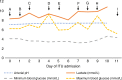
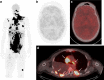
A 47-year-old man with metastatic melanoma presented with refractory hyperlactaemic acidosis following the first dose of the mono-carboxylase transporter 1 inhibitor AZD3965 within a "first time in man" clinical trial. The mechanism of the agent and the temporal relationship suggested that this event was potentially drug related and recruitment was suspended. However, urinary metabolomics showed extensive abnormalities even prior to drug administration, leading to investigations for an underlying metabolic disorder. The lack of clinical symptoms from the elevated lactate and low blood glucose suggested a diagnosis of "hyper-Warburgism", where the high tumour burden was associated with extensive glucose uptake and lactate efflux from malignant cells, and the subsequent impact on blood biochemistry. This was supported by an FDG-PET scan showing extensive glucose uptake in numerous metastases and lack of uptake in the brain. A review of the literature showed 16 case reports of "hyper-Warburgism" in non-haematological malignancies, none of them with melanoma, with most associated with a poor outcome. The patient was treated symptomatically, but died 2 months later. The development of AZD3965 continues with the exclusion of patients with elevated plasma lactate at screening added to the protocol as a safety measure.Trial identification number ClinicalTrials.Gov. NCT01791595.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- de Groot R, Sprenger RA, Imholz AL, Gerding MN. Type B lactic acidosis in solid malignancies. Neth. J. Med. 2011;69:120–123. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

