Imipramine and Venlafaxine Differentially Affect Primary Glial Cultures of Prenatally Stressed Rats
- PMID: 32076407
- PMCID: PMC7006619
- DOI: 10.3389/fphar.2019.01687
Imipramine and Venlafaxine Differentially Affect Primary Glial Cultures of Prenatally Stressed Rats
Abstract
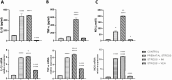
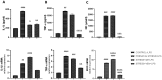
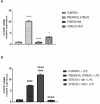
Here, we examine the effects of prenatal administration of two antidepressants-imipramine (IMI) and venlafaxine (VEN)-on morphology and activity of a primary glial culture. Microglia are targeted by antidepressants used for antenatal depression and are important regulators of central nervous system development. In this study, female Wistar rats were assigned to one of four groups: a control group that received water ad libitum (1), and groups that received additionally once daily either water (2), IMI (10 mg/kg) (3), or VEN (20 mg/kg) (4) by oral gavage from gestation day 7 to 22. Oral gavage administration induced prenatal stress. Cell cultures were obtained from the brains of 1-day-old pups. Prenatal stress caused a disturbance of sensorimotor function in pups. Prenatal stress also produced alterations in the glial cultures, specifically, an increased percentage of microglia in the mixed glial cultures and an increased percentage of dead cells. Moreover, increased levels of IL1-β, TNF-α, NO, and an increased expression of CX3CR1 mRNA were found in microglia. However, the ratio of Bax/Bcl2 mRNA was reduced. Prenatal stress increased the vulnerability of microglia to lipopolysaccharide (LPS). The mixed glial culture derived from pups exposed to IMI showed greater morphological changes and the highest percentage of microglia. Microglia were characterized by the largest increase in the production of pro-inflammatory cytokines and NO, and the greatest reduction in the expression of CX3CR1 mRNA. Exposure to IMI reduced the effects of LPS on IL-1β production and Bax/Bcl2 mRNA, and exacerbated the effects of LPS on CX3CR1 mRNA expression. Prenatal administration of VEN induced protective effects on microglia, as measured by all studied parameters. Taken together, our data suggest that, by disturbing microglia function, exposure to even mild forms of chronic prenatal stress may predispose individuals to psychiatric or neurodevelopmental disorders. These data also indicate that chronic mild stress sensitizes microglia to immune challenges, which may lead to enhanced neuronal damage in the embryonic brain. The observed detrimental effects of IMI on microglial activity under conditions of prenatal stress may help to explain the teratogenic effects of IMI reported in the literature.
Keywords: imipramine; prenatal; primary glial cell cultures; rat; stress; venlafaxine.
Copyright © 2020 Obuchowicz, Bielecka-Wajdman, Zieliński, Machnik, Gołyszny and Ludyga.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials

