ZNF143 Suppresses Cell Apoptosis and Promotes Proliferation in Gastric Cancer via ROS/p53 Axis
- PMID: 32076462
- PMCID: PMC7017572
- DOI: 10.1155/2020/5863178
ZNF143 Suppresses Cell Apoptosis and Promotes Proliferation in Gastric Cancer via ROS/p53 Axis
Abstract
Aim: This study was aimed at identifying the role of zinc finger protein 143 (ZNF143) in gastric cancer (GC) progression.
Methods: The impact of ZNF143 on the proliferation ability and apoptosis of GC cells was detected. The expression of ZNF143 and related targeted genes was determined using Western blot analysis. The reactive oxygen species (ROS) level of GC cells was examined using the ROS generation assay. The role of ZNF143 in the proliferation of GC cells in vivo was examined using tumor xenograft assay.
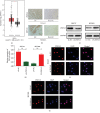
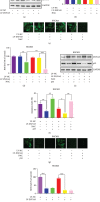
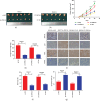
Results: The ectopic overexpression of ZNF143 promoted the proliferation of GC cells, while its knockdown reduced the effect in vitro. The downregulation of ZNF143 facilitated cell apoptosis. ZNF143 decreased the ROS level in GC cells, resulting in the reduction of cell apoptosis. Transfection with p53 reversed the antiapoptotic effect of ZNF143, while pifithrin-α, a specific inhibitor of p53, reduced the apoptosis in ZNF143-knockdown GC cells. However, p53 had no influence on the ROS level in GC cells. p53 played a key role in inhibiting ROS generation in GC cells, thereby inhibiting apoptosis. The transplanted tumor weight and volume were higher in the ZNF143-overexpressed group than in the ZNF143-knockdown group in vivo was examined using tumor xenograft assay.
Conclusion: ZNF143, as a tumor oncogene, promoted the proliferation of GC cells both in vitro and in vivo, indicating that ZNF143 might function as a novel target for GC therapy.in vitro. The downregulation of ZNF143 facilitated cell apoptosis. ZNF143 decreased the ROS level in GC cells, resulting in the reduction of cell apoptosis. Transfection with p53 reversed the antiapoptotic effect of ZNF143, while pifithrin-in vivo was examined using tumor xenograft assay.
Copyright © 2020 Yi Zhang et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Wang F., Wei X. L., Wang F. H., et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Annals of Oncology. 2019;30(9):1479–1486. doi: 10.1093/annonc/mdz197. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

