Tranexamic acid in traumatic brain injury: an explanatory study nested within the CRASH-3 trial
- PMID: 32076783
- PMCID: PMC7851008
- DOI: 10.1007/s00068-020-01316-1
Tranexamic acid in traumatic brain injury: an explanatory study nested within the CRASH-3 trial
Abstract
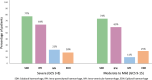
Purpose: The CRASH-3 trial is a randomised trial of tranexamic acid (TXA) on death and disability in patients with traumatic brain injury (TBI). It is based on the hypothesis that early TXA treatment can prevent deaths from post-traumatic intracranial bleeding. The results showed that timely TXA treatment reduces head injury deaths in patients with reactive pupils and those with a mild to moderate GCS at baseline. We examined routinely collected CT scans in a sample of 1767 CRASH-3 trial patients to explore if, why, and how patients are affected by TXA.
Methods: The CRASH-3 IBMS is an explanatory study nested within the CRASH-3 trial. We measured the volume of intracranial bleeding on CT scans using established methods (e.g. ABC/2).
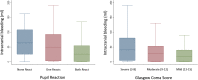
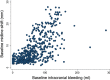
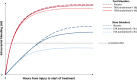
Results: Patients with any un-reactive pupil had a median intracranial bleeding volume of 60 ml (IQR 18-101 ml) and patients with reactive pupils had a median volume of 26 ml (IQR 1-55 ml). Patients with severe GCS had median intracranial bleeding volume of 37 ml (IQR 3-75 ml) and patients with moderate to mild GCS had a median volume of 26 ml (IQR 0.4-50 ml). For every hour increase from injury to the baseline scan, the risk of new bleeding on a further scan decreased by 12% (adjusted RR = 0.88 [95% CI 0.80-0.96], p = 0.0047).
Conclusion: Patients with reactive pupils and/or mild to moderate GCS may have benefited from TXA in the CRASH-3 trial because they had less intracranial bleeding at baseline. However, because bleeding occurs soon after injury, treatment delay reduces the benefit of TXA.
Keywords: Tranexamic acid; Traumatic brain injury.
Conflict of interest statement
Abda Mahmood, Adrian Boyle, Antonio Belli, Caroline Leech, Darin Wong, David Davies, Fatahul Laham Mohamed, Haleema Shakur-Still, Hamzah Lotfi, Ian Roberts, Jason Kendall, Kelly Needham, Mark Wilson, Melanie Darwent, Phillip Hopkins, Phil Moss, Sabariah Faizah Jamaluddin and Tim Harris have no conflicts of interest to declare. Antonio Belli was in receipt of a grant from the National Institute for Health Research during the conduct of the study.
Figures


Comment in
-
CRASH 3: a monumental effort with minimal gain.Eur J Trauma Emerg Surg. 2021 Feb;47(1):269-271. doi: 10.1007/s00068-020-01424-y. Epub 2020 Jun 24. Eur J Trauma Emerg Surg. 2021. PMID: 32583071 No abstract available.
References
-
- The CRASH-3 trial collaborators Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): a randomised, placebo-controlled trial. Lancet. 2019;394(10210):1713–1723. doi: 10.1016/S0140-6736(19)32233-0. - DOI - PMC - PubMed
-
- Gayet-Ageron A, Prieto-Merino D, Ker K, Shakur H, Ageron FX, Roberts I, et al. Effect of treatment delay on the effectiveness and safety of antifibrinolytics in acute severe haemorrhage: a meta-analysis of individual patient-level data from 40,138 bleeding patients. Lancet. 2018;391(10116):125–132. doi: 10.1016/S0140-6736(17)32455-8. - DOI - PMC - PubMed
-
- Dewan Y, Komolafe EO, Mejia-Mantilla JH, Perel P, Roberts I, Shakur H, et al. CRASH-3—tranexamic acid for the treatment of significant traumatic brain injury: study protocol for an international randomized, double-blind, placebo-controlled trial. Trials. 2012;13:87. doi: 10.1186/1745-6215-13-87. - DOI - PMC - PubMed
-
- Mahmood A, Roberts I, Shakur H. A nested mechanistic sub-study into the effect of tranexamic acid versus placebo on intracranial haemorrhage and cerebral ischaemia in isolated traumatic brain injury: study protocol for a randomised controlled trial (CRASH-3 Trial Intracranial Bleeding Mechanistic Sub-Study [CRASH-3 IBMS]) Trials. 2017;18(1):330. doi: 10.1186/s13063-017-2073-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

