The impact of patient characteristics on enzalutamide pharmacokinetics and how this relates to treatment toxicity and efficacy in metastatic prostate cancer patients
- PMID: 32076807
- PMCID: PMC7125069
- DOI: 10.1007/s00280-020-04039-7
The impact of patient characteristics on enzalutamide pharmacokinetics and how this relates to treatment toxicity and efficacy in metastatic prostate cancer patients
Abstract
Purpose: The aim of the study is to investigate the influence of patient characteristics, age and body mass index (BMI), on pharmacokinetics of enzalutamide, and to study the relationships between drug exposure and enzalutamide efficacy and toxicity, in mCRPC patients.
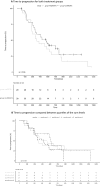
Methods: Data were collected in a longitudinal cohort study (ANDROPS) and a prospective observational study (ILUMINATE), both in mCRPC patients treated with enzalutamide. To investigate the influence of age and BMI on exposure, enzalutamide and N-desmethylenzalutamide levels were compared by ANOVA. To investigate the relation of exposure versus time to progression (TTP), the sum plasma levels were divided into quartiles and compared by Kaplan-Meier analysis. To assess the relation of exposure with fatigue, plasma levels in patients experiencing fatigue vs. no fatigue were compared by and independent t test.
Results: Data of 68 mCRPC patients were included for analysis. Plasma levels were not different for age or BMI. No difference in TTP between both studies was observed (383 days (95% CI 287-859), and 567 days (95% CI 351-NR), p = 0.36). Kaplan-Meier analysis of quartiles of sum levels showed no difference for TTP. Fatigue was reported by 22 patients, no difference in sum plasma levels was observed between patients with and without fatigue.
Conclusions: We observed that age and BMI did not influence systemic exposure in patients treated with enzalutamide. No relation of exposure with efficacy or fatigue was observed. Further research using enzalutamide at a lower dose is needed to understand the relation of enzalutamide exposure and fatigue.
Keywords: Enzalutamide; Fatigue; Metastatic castration-resistant prostate cancer; Pharmacokinetics.
Conflict of interest statement
Dr. van Erp reports grants from ZonMW, during the conduct of the study; grants from Astellas, grants from Janssen-Cilag, outside the submitted work; Dr. van Oort reports grants from ZonMw, during the conduct of the study; personal fees and other from Astellas, personal fees and other from Janssen, outside the submitted work; Dr Mehra reports personal fees from Roche, MSD, BMS, Bayer, Astellas and Janssen’, grants from Astellas, Janssen Pfizer, Roche and Sanofi’ Genzyme, work and other from Astellas and MSD, outside the submitted work. The other authors have nothing to disclose.
Figures
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68(6):394–424. - PubMed
-
- Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA, TAX Investigators Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. - DOI - PubMed
-
- Beer TM, Armstrong AJ, Rathkopf D, Loriot Y, Sternberg CN, Higano CS, Iversen P, Evans CP, Kim CS, Kimura G, Miller K, Saad F, Bjartell AS, Borre M, Mulders P, Tammela TL, Parli T, Sari S, van Os S, Theeuwes A, Tombal B. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the phase 3 prevail study. Eur Urol. 2017;71(2):151–154. doi: 10.1016/j.eururo.2016.07.032. - DOI - PMC - PubMed
-
- Berthold DR, Pond GR, Roessner M, de Wit R, Eisenberger M, Tannock AI, Investigators TAX Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin Cancer Res. 2008;14(9):2763–2767. doi: 10.1158/1078-0432.CCR-07-0944. - DOI - PubMed
-
- Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Flechon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS, Investigators A. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical