Clinical implementation of pharmacogenomics via a health system-wide research biobank: the University of Colorado experience
- PMID: 32077359
- PMCID: PMC7226704
- DOI: 10.2217/pgs-2020-0007
Clinical implementation of pharmacogenomics via a health system-wide research biobank: the University of Colorado experience
Abstract
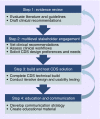
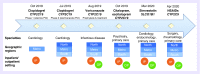
In recent years, the genomics community has witnessed the growth of large research biobanks, which collect DNA samples for research purposes. Depending on how and where the samples are genotyped, biobanks also offer the potential opportunity to return actionable genomic results to the clinical setting. We developed a preemptive clinical pharmacogenomic implementation initiative via a health system-wide research biobank at the University of Colorado. Here, we describe how preemptive return of clinical pharmacogenomic results via a research biobank is feasible, particularly when coupled with strong institutional support to maximize the impact and efficiency of biobank resources, a multidisciplinary implementation team, automated clinical decision support tools, and proactive strategies to engage stakeholders early in the clinical decision support tool development process.
Keywords: biobank; implementation; personalized medicine; pharmacogenetics; pharmacogenomics; precision medicine.
Conflict of interest statement
This work was supported, in part, by NIH grants R01HL104608-01 (KCB), K08HL125725 (DPK), and R35GM124939 (AAM). Contents are the authors' sole responsibility and do not necessarily represent official NIH views. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
