Clinical imaging of cardiovascular inflammation
- PMID: 32077666
- PMCID: PMC7145733
- DOI: 10.23736/S1824-4785.20.03228-8
Clinical imaging of cardiovascular inflammation
Abstract
Cardiovascular disease due to atherosclerosis is the number one cause of morbidity and mortality worldwide. In the past twenty years, compelling preclinical and clinical data have indicated that a maladaptive inflammatory response plays a crucial role in the development of atherosclerosis initiation and progression in the vasculature, all the way to the onset of life-threatening cardiovascular events. Furthermore, inflammation is key to heart and brain damage and healing after myocardial infarction or stroke. Recent evidence indicates that this interplay between the vasculature, organs target of ischemia and the immune system is mediated by the activation of hematopoietic organs (bone marrow and spleen). In this evolving landscape, non-invasive imaging is becoming more and more essential to support either mechanistic preclinical studies to investigate the role of inflammation in cardiovascular disease (CVD), or as a translational tool to quantify inflammation in the cardiovascular system and hematopoietic organs in patients. In this review paper, we will describe the clinical applications of non-invasive imaging to quantify inflammation in the vasculature, infarcted heart and brain, and hematopoietic organs in patients with cardiovascular disease, with specific focus on [18F]FDG PET and other novel inflammation-specific radiotracers. Furthermore, we will briefly describe the most recent clinical applications of other imaging techniques such as MRI, SPECT, CT, CEUS and OCT in this arena.
Conflict of interest statement
Figures
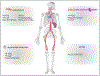
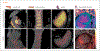
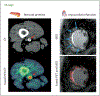
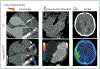
References
-
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers 2019;5:56. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

