Current and novel radiopharmaceuticals for imaging cardiovascular inflammation
- PMID: 32077667
- PMCID: PMC7985841
- DOI: 10.23736/S1824-4785.20.03230-6
Current and novel radiopharmaceuticals for imaging cardiovascular inflammation
Abstract
Cardiovascular disease (CVD) remains the leading cause of death worldwide despite advances in diagnostic technologies and treatment strategies. The underlying cause of most CVD is atherosclerosis, a chronic disease driven by inflammatory reactions. Atherosclerotic plaque rupture could cause arterial occlusion leading to ischemic tissue injuries such as myocardial infarction (MI) and stroke. Clinically, most imaging modalities are based on anatomy and provide limited information about the on-going molecular activities affecting the vulnerability of atherosclerotic lesion for risk stratification of patients. Thus, the ability to differentiate stable plaques from those that are vulnerable is an unmet clinical need. Of various imaging techniques, the radionuclide-based molecular imaging modalities including positron emission tomography and single-photon emission computerized tomography provide superior ability to noninvasively visualize molecular activities in vivo and may serve as a useful tool in tackling this challenge. Moreover, the well-established translational pathway of radiopharmaceuticals may also facilitate the translation of discoveries from benchtop to clinical investigation in contrast to other imaging modalities to fulfill the goal of precision medicine. The relationship between inflammation occurring within the plaque and its proneness to rupture has been well documented. Therefore, an active effort has been significantly devoted to develop radiopharmaceuticals specifically to measure CVD inflammatory status, and potentially elucidate those plaques which are prone to rupture. In the following review, molecular imaging of inflammatory biomarkers will be briefly discussed.
Conflict of interest statement
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Figures
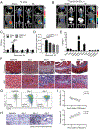

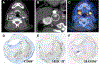
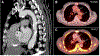
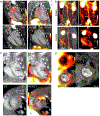
Similar articles
-
Inflammation imaging to define vulnerable plaque or vulnerable patient.Q J Nucl Med Mol Imaging. 2020 Mar;64(1):21-34. doi: 10.23736/S1824-4785.20.03231-8. Epub 2020 Feb 18. Q J Nucl Med Mol Imaging. 2020. PMID: 32077668 Review.
-
PET and SPECT imaging of apoptosis in vulnerable atherosclerotic plaques with radiolabeled Annexin A5.Q J Nucl Med Mol Imaging. 2009 Feb;53(1):26-34. Q J Nucl Med Mol Imaging. 2009. PMID: 19182725 Review.
-
The clinical value of quantitative cardiovascular molecular imaging: a step towards precision medicine.Br J Radiol. 2023 Dec;96(1152):20230704. doi: 10.1259/bjr.20230704. Epub 2023 Oct 24. Br J Radiol. 2023. PMID: 37786997 Free PMC article. Review.
-
Current diagnostic modalities for vulnerable plaque detection.Curr Pharm Des. 2007;13(10):995-1001. doi: 10.2174/138161207780487511. Curr Pharm Des. 2007. PMID: 17430163 Review.
-
Nuclear Imaging: Focus on Vascular Probes.Arterioscler Thromb Vasc Biol. 2019 Jul;39(7):1369-1378. doi: 10.1161/ATVBAHA.119.312586. Epub 2019 May 23. Arterioscler Thromb Vasc Biol. 2019. PMID: 31242032 Review.
Cited by
-
Delineating the Role of Macrophages in Cardiovascular Disease: How Specific Do We Need to Be?Circ Cardiovasc Imaging. 2020 Oct;13(10):e011605. doi: 10.1161/CIRCIMAGING.120.011605. Epub 2020 Oct 20. Circ Cardiovasc Imaging. 2020. PMID: 33076697 Free PMC article. No abstract available.
-
Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade.Int J Mol Sci. 2022 Apr 30;23(9):5023. doi: 10.3390/ijms23095023. Int J Mol Sci. 2022. PMID: 35563414 Free PMC article.
-
Recent Advances in Cardiovascular Diseases Research Using Animal Models and PET Radioisotope Tracers.Int J Mol Sci. 2022 Dec 26;24(1):353. doi: 10.3390/ijms24010353. Int J Mol Sci. 2022. PMID: 36613797 Free PMC article. Review.
-
The Latest Advances in Imaging Crosstalk Between the Immune System and Fibrosis in Cardiovascular Disease.J Nucl Med. 2021 Oct;62(10):1341-1346. doi: 10.2967/jnumed.120.255539. Epub 2021 Apr 16. J Nucl Med. 2021. PMID: 33863824 Free PMC article. Review.
-
Fantastic voyage: Catheter-based quantification of tracer distribution on a miniature scale.J Nucl Cardiol. 2022 Apr;29(2):677-679. doi: 10.1007/s12350-020-02379-8. Epub 2020 Oct 6. J Nucl Cardiol. 2022. PMID: 33025474 Free PMC article. No abstract available.
References
-
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart Disease and Stroke Statistics - 2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528. - PubMed
-
- Organization WH. Global status report on noncommunicable diseases 2014. World Health Organization, 2014.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

