Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial
- PMID: 32077891
- PMCID: PMC7042914
- DOI: 10.1001/jamaoncol.2019.6731
Phase 1 Trial of Pembrolizumab Administered Concurrently With Chemoradiotherapy for Locally Advanced Non-Small Cell Lung Cancer: A Nonrandomized Controlled Trial
Abstract
Importance: Consolidative programmed death ligand-1 (PD-L) inhibition after chemoradiotherapy improves overall survival and progression-free survival (PFS) for stage III non-small cell lung cancer (NSCLC) and requires safety evaluation for incorporation of programmed cell death 1 (PD-1) inhibition at the onset of chemoradiotherapy.
Objective: To determine the safety and tolerability of PD-1 inhibition concurrently with definitive chemoradiotherapy for NSCLC.
Design, setting, and participants: This phase 1 prospective multicenter nonrandomized controlled trial using a 3 plus 3 design was performed from August 30, 2016, to October 24, 2018, with a median follow-up of 16.0 (95% CI, 12.0-22.6) months and data locked on July 25, 2019. Twenty-one participants had locally advanced, unresectable, stage III NSCLC as determined by multidisciplinary review, Eastern Cooperative Oncology Group performance status 0 or 1, and adequate hematologic, renal, and hepatic function. Data were analyzed from October 17, 2016, to July 19, 2019.
Interventions: Pembrolizumab was combined with concurrent chemoradiotherapy (weekly carboplatin and paclitaxel with 60 Gy of radiation in 2 Gy per d). Dose cohorts evaluated included full-dose pembrolizumab (200 mg intravenously every 3 weeks) 2 to 6 weeks after chemoradiotherapy (cohort 1); reduced-dose pembrolizumab (100 mg intravenously every 3 weeks) starting day 29 of chemoradiotherapy (cohort 2); full-dose pembrolizumab starting day 29 of chemoradiotherapy (cohort 3); reduced-dose pembrolizumab starting day 1 of chemoradiotherapy (cohort 4); and full-dose pembrolizumab starting day 1 of chemoradiotherapy (cohort 5). A safety expansion cohort of 6 patients was planned based on the maximum tolerated dose of pembrolizumab. Dose-limiting toxic effects were defined as pneumonitis of at least grade 4 within cycle 1 of pembrolizumab treatment.
Main outcomes and measures: Safety and tolerability of PD-1 inhibition with chemoradiotherapy for NSCLC. Secondary outcomes included PFS and pneumonitis rates.
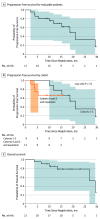
Results: Among the 21 patients included in the analysis (11 female [52%]; median age, 69.5 [range, 53.0-85.0] years), no dose-limiting toxic effects in any cohort were observed. One case of grade 5 pneumonitis occurred in the safety expansion cohort with the cohort 5 regimen. Immune-related adverse events of at least grade 3 occurred in 4 patients (18%). Median PFS for patients who received at least 1 dose of pembrolizumab (n = 21) was 18.7 (95% CI, 11.8-29.4) months, and 6- and 12-month PFS were 81.0% (95% CI, 64.1%-97.7%) and 69.7% (95% CI, 49.3%-90.2%), respectively. Median PFS for patients who received at least 2 doses of pembrolizumab (n = 19) was 21.0 (95% CI, 15.3 to infinity) months.
Conclusions and relevance: These findings suggest that combined treatment with PD-1 inhibitors and chemoradiotherapy for stage III NSCLC is tolerable, with promising PFS of 69.7% at 12 months, and requires further study.
Trial registration: ClinicalTrials.gov Identifier: NCT02621398.
Conflict of interest statement
Figures
Comment in
-
Assessing Expression of PD-L1 in Tumor-Associated Macrophages-Reply.JAMA Oncol. 2020 Oct 1;6(10):1634-1635. doi: 10.1001/jamaoncol.2020.2722. JAMA Oncol. 2020. PMID: 32761104 No abstract available.
-
Assessing Expression of PD-L1 in Tumor-Associated Macrophages.JAMA Oncol. 2020 Oct 1;6(10):1634. doi: 10.1001/jamaoncol.2020.2698. JAMA Oncol. 2020. PMID: 32761136 No abstract available.
-
Advances in immunotherapy for stage III non-small cell lung cancer: moving immune checkpoint inhibitors to the front lines concurrently with chemoradiotherapy?J Thorac Dis. 2020 Aug;12(8):4549-4552. doi: 10.21037/jtd-20-1672. J Thorac Dis. 2020. PMID: 32944372 Free PMC article. No abstract available.
References
-
- Howlader NNA, Noone AM, Krapcho M, et al. , et al. SEER Cancer Statistics Review, 1975-2016, National Cancer Institute. Bethesda, MD. https://seer.cancer.gov/csr/1975_2016/. Published April 2019. Accessed April 1, 2019.
-
- Bradley JD, Paulus R, Komaki R, et al. . Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187-199. doi:10.1016/S1470-2045(14)71207-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

