Genome-wide assessment of genetic risk for systemic lupus erythematosus and disease severity
- PMID: 32077931
- PMCID: PMC7322569
- DOI: 10.1093/hmg/ddaa030
Genome-wide assessment of genetic risk for systemic lupus erythematosus and disease severity
Abstract
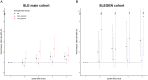
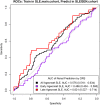
Using three European and two Chinese genome-wide association studies (GWAS), we investigated the performance of genetic risk scores (GRSs) for predicting the susceptibility and severity of systemic lupus erythematosus (SLE), using renal disease as a proxy for severity. We used four GWASs to test the performance of GRS both cross validating within the European population and between European and Chinese populations. The performance of GRS in SLE risk prediction was evaluated by receiver operating characteristic (ROC) curves. We then analyzed the polygenic nature of SLE statistically. We also partitioned patients according to their age-of-onset and evaluated the predictability of GRS in disease severity in each age group. We found consistently that the best GRS in the prediction of SLE used SNPs associated at the level of P < 1e-05 in all GWAS data sets and that SNPs with P-values above 0.2 were inflated for SLE true positive signals. The GRS results in an area under the ROC curve ranging between 0.64 and 0.72, within European and between the European and Chinese populations. We further showed a significant positive correlation between a GRS and renal disease in two independent European GWAS (Pcohort1 = 2.44e-08; Pcohort2 = 0.00205) and a significant negative correlation with age of SLE onset (Pcohort1 = 1.76e-12; Pcohort2 = 0.00384). We found that the GRS performed better in the prediction of renal disease in the 'later onset' compared with the 'earlier onset' group. The GRS predicts SLE in both European and Chinese populations and correlates with poorer prognostic factors: young age-of-onset and lupus nephritis.
© The Author(s) 2020. Published by Oxford University Press.
Figures




References
-
- Kaul A., Gordon C., Crow M.K., Touma Z., Urowitz M.B., Vollenhoven R., Ruiz-Irastorza G. and Hughes G. (2016) Systemic lupus erythematosus. Nat. Rev. Dis. Primers., 2, 16039. - PubMed
-
- Feldman C.H., Hiraki L.T., Liu J., Fischer M.A., Solomon D.H., Alarcon G.S., Winkelmayer W.C. and Costenbader K.H. (2013) Epidemiology and sociodemographics of systemic lupus erythematosus and lupus nephritis among US adults with medicaid coverage, 2000-2004. Arthritis Rheum., 65, 753–763. - PMC - PubMed
-
- Peschken C.A., Katz S.J., Silverman E., Pope J.E., Fortin P.R., Pineau C., Smith C.D., Arbillaga H.O., Gladman D.D., Urowitz M. et al. (2009) The 1000 Canadian faces of lupus: determinants of disease outcome in a large multiethnic cohort. J. Rheumatol., 36, 1200–1208. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

