Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients
- PMID: 32078016
- PMCID: PMC11027674
- DOI: 10.1007/s00262-020-02496-w
Tumor-associated antigen-based personalized dendritic cell vaccine in solid tumor patients
Abstract
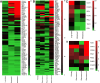
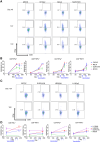
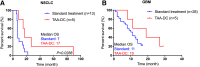
Tumor-associated antigens (TAAs) have been tested in various clinical trials in cancer treatment but the patterns of specific T cell response to personalized TAA immunization remains to be fully understood. We report antigen-specific T cell responses in patients immunized with dendritic cell vaccines pulsed with personalized TAA panels. Tumor samples from patients were first analyzed to identify overexpressed TAAs. Autologous DCs were then transfected with pre-manufactured mRNAs encoding the full-length TAAs, overexpressed in the patients' tumors. Patients with glioblastoma multiforme (GBM) or advanced lung cancer received DC vaccines transfected with personalized TAA panels, in combination with low-dose cyclophosphamide, poly I:C, imiquimod and anti-PD-1 antibody. Antigen-specific T cell responses were measured. Safety and efficacy were evaluated. A total of ten patients were treated with DC vaccines transfected with personalized TAA panels containing 3-13 different TAAs. Among the seven patients tested for anti-TAA T cell responses, most of the TAAs induced antigen-specific CD4+ and/or CD8+ T cell responses, regardless of their expression levels in the tumor tissues. No Grade III/IV adverse events were observed among these patients. Furthermore, the treated patients were associated with favorable overall survival when compared to patients who received standard treatment in the same institution. Personalized TAA immunization-induced-specific CD4+ and CD8+ T cell responses without obvious autoimmune adverse events and was associated with favorable overall survival. These results support further studies on DC immunization with personalized TAA panels for combined immunotherapeutic regimens in solid tumor patients.Trial registration ClinicalTrials.gov, NCT02709616 (March, 2016), NCT02808364 (June 2016), NCT02808416 (June, 2016).
Keywords: CD4+ T cell response; CD8+ T cell response; DC vaccine; Glioblastoma multiforme; Non-small cell lung cancer; Personalization; Tumor-associated antigen.
Conflict of interest statement
You-Wen He is a co-founder of Beijing Tricision Biotherapeutics. Huaxin Liao is a co-founder of Guangzhou Trinomab Inc. Shi-You Li is a co-founder of Beijing Tricision Biotherapeutics Inc. Sheng-Nan Sun and Jun Jiang are full-time employees of Beijing Tricision Biotherapeutics Inc. Yun-Peng Xiao is full-time employee of Guangzhou Trinomab Biotechnology Inc.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

