Long-term outcomes after contaminated complex abdominal wall reconstruction
- PMID: 32078080
- PMCID: PMC7210226
- DOI: 10.1007/s10029-020-02124-7
Long-term outcomes after contaminated complex abdominal wall reconstruction
Abstract
Purpose: Complex abdominal wall repair (CAWR) in a contaminated operative field is a challenge. Available literature regarding long-term outcomes of CAWR comprises studies that often have small numbers and heterogeneous patient populations. This study aims to assess long-term outcomes of modified-ventral hernia working group (VHWG) grade 3 repairs. Because the relevance of hernia recurrence (HR) as the primary outcome for this patient group is contentious, the need for further hernia surgery (FHS) was also assessed in relation to long-term survival.
Methods: A retrospective cohort study with a single prospective follow-up time-point nested in a consecutive series of patients undergoing CAWR in two European national intestinal failure centers.
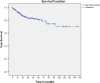
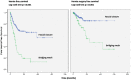
Results: In long-term analysis, 266 modified VHWG grade 3 procedures were included. The overall HR rate was 32.3%. The HR rates for non-crosslinked biologic meshes and synthetic meshes when fascial closure was achieved were 20.3% and 30.6%, respectively. The rates of FHS were 7.2% and 16.7%, and occurred only within the first 3 years. Bridged repairs showed poorer results (fascial closure 22.9% hernia recurrence vs bridged 57.1% recurrence). Overall survival was relatively good with 80% en 70% of the patients still alive after 5 and 10 years, respectively. In total 86.6% of the patients remained free of FHS.
Conclusions: In this study of contaminated CAWR, non-crosslinked biologic mesh shows better results than synthetic mesh. Bridging repairs with no posterior and/or anterior fascial closure have a higher recurrence rate. The overall survival was good and the majority of patients remained free of additional hernia surgery.
Keywords: Complex; Contamination; Hernia repair; Mesh; Outcomes.
Conflict of interest statement
JDH, FEEdV, JJMC, CAL, YM, OvR, OL, MCO, PJT, WAB, JC, GBH and JW declare no conflict of interest directly related to the current work; CJV declares no conflict of interest directly related to the current work and declares consultancy advisor to Acelity and paid lecture for Allergan. MAB declares no conflict of interest directly related to the current work and reports institutional research grants from Baxter, Mylan, Ipsen, Acelity/KCI, Bard, LifeCell and Johnson & Johnson/Ethicon and New Compliance; and is a speaker or advisory board member for Acelity/KCI, Bard, LifeCell/Allergan, Gore, Bard, Smith&Nephew and Johnson & Johnson/Ethicon.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

