Sequential Targeting in Crosslinking Nanotheranostics for Tackling the Multibarriers of Brain Tumors
- PMID: 32078198
- PMCID: PMC7148201
- DOI: 10.1002/adma.201903759
Sequential Targeting in Crosslinking Nanotheranostics for Tackling the Multibarriers of Brain Tumors
Abstract
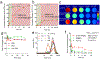
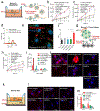
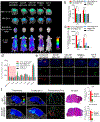
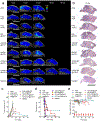
The efficacy of therapeutics for brain tumors is seriously hampered by multiple barriers to drug delivery, including severe destabilizing effects in the blood circulation, the blood-brain barrier/blood-brain tumor barrier (BBB/BBTB), and limited tumor uptake. Here, a sequential targeting in crosslinking (STICK) nanodelivery strategy is presented to circumvent these important physiological barriers to improve drug delivery to brain tumors. STICK nanoparticles (STICK-NPs) can sequentially target BBB/BBTB and brain tumor cells with surface maltobionic acid (MA) and 4-carboxyphenylboronic acid (CBA), respectively, and simultaneously enhance nanoparticle stability with pH-responsive crosslinkages formed by MA and CBA in situ. STICK-NPs exhibit prolonged circulation time (17-fold higher area under curve) than the free agent, allowing increased opportunities to transpass the BBB/BBTB via glucose-transporter-mediated transcytosis by MA. The tumor acidic environment then triggers the transformation of the STICK-NPs into smaller nanoparticles and reveals a secondary CBA targeting moiety for deep tumor penetration and enhanced uptake in tumor cells. STICK-NPs significantly inhibit tumor growth and prolong the survival time with limited toxicity in mice with aggressive and chemoresistant diffuse intrinsic pontine glioma. This formulation tackles multiple physiological barriers on-demand with a simple and smart STICK design. Therefore, these features allow STICK-NPs to unleash the potential of brain tumor therapeutics to improve their treatment efficacy.
Keywords: blood-brain barrier; diffuse intrinsic pontine glioma; pH response; sequential targeting.
© 2020 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


References
-
- Mohammad F, Weissmann S, Leblanc B, Pandey DP, Hojfeldt JW, Comet I, Zheng CQ, Johansen JV, Rapin N, Porse BT, Tvardovskiy A, Jensen ON, Olaciregui NG, Lavarino C, Sunol M, de Torres C, Mora J, Carcaboso AM, Helin K, Nat. Med 2017, 23, 483. - PubMed
-
- Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, Morrison A, Lewis P, Bouffet E, Bartels U, Zuccaro J, Agnihotri S, Rya S, Barszczyk M, Chornenkyy Y, Bourgey M, Bourque G, Montpetit A, Cordero F, Castelo-Branco P, Mangere J, Tabori U, Ching K, Huang A, Taylor KR, Mackay A, Bendell AE, Nazarian J, Fangusaro JR, Karajannis MA, Zagzag D, Foreman NK, Donson A, Hegert JV, Smith A, Chan J, Lafay-Cousin L, Dunn S, Hukin J, Dunham C, Scheinemann K, Michaud J, Zelcer S, Ramsay D, Cain J, Brennan C, Souweidane MM, Jones C, Allis CD, Brudno M, Becher O, Hawkins C, Nat. Genet 2014, 46, 451. - PMC - PubMed
-
- Zhu QW, Chen XJ, Xu X, Zhang Y, Zhang C, Mo R, Adv. Funct. Mater 2018, 28.
-
- Wu D, Qin M, Xu D, Wang L, Liu C, Ren J, Zhou G, Chen C, Yang F, Li Y, Adv. Mater 2019, 1807557; - PMC - PubMed
- He QG, Liu J, Liang J, Liu XP, Li W, Liu Z, Ding ZY, Tuo D, Cells 2018, 7; - PMC - PubMed
- Chen WS, Ouyang J, Yi XY, Xu Y, Niu CC, Zhang WY, Wang LQ, Sheng JP, Deng L, Liu YN, Guo SJ, Adv. Mater 2018, 30. - PubMed

