BIVV001, a new class of factor VIII replacement for hemophilia A that is independent of von Willebrand factor in primates and mice
- PMID: 32078672
- PMCID: PMC7180082
- DOI: 10.1182/blood.2019001292
BIVV001, a new class of factor VIII replacement for hemophilia A that is independent of von Willebrand factor in primates and mice
Abstract
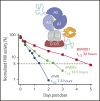
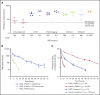
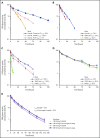
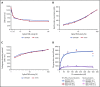
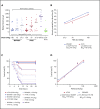
Factor VIII (FVIII) replacement products enable comprehensive care in hemophilia A. Treatment goals in severe hemophilia A are expanding beyond low annualized bleed rates to include long-term outcomes associated with high sustained FVIII levels. Endogenous von Willebrand factor (VWF) stabilizes and protects FVIII from degradation and clearance, but it also subjects FVIII to a half-life ceiling of ∼15 to 19 hours. Increasing recombinant FVIII (rFVIII) half-life further is ultimately dependent upon uncoupling rFVIII from endogenous VWF. We have developed a new class of FVIII replacement, rFVIIIFc-VWF-XTEN (BIVV001), that is physically decoupled from endogenous VWF and has enhanced pharmacokinetic properties compared with all previous FVIII products. BIVV001 was bioengineered as a unique fusion protein consisting of a VWF-D'D3 domain fused to rFVIII via immunoglobulin-G1 Fc domains and 2 XTEN polypeptides (Amunix Pharmaceuticals, Inc, Mountain View, CA). Plasma FVIII half-life after BIVV001 administration in mice and monkeys was 25 to 31 hours and 33 to 34 hours, respectively, representing a three- to fourfold increase in FVIII half-life. Our results showed that multifaceted protein engineering, far beyond a few amino acid substitutions, could significantly improve rFVIII pharmacokinetic properties while maintaining hemostatic function. BIVV001 is the first rFVIII with the potential to significantly change the treatment paradigm for severe hemophilia A by providing optimal protection against all bleed types, with less frequent doses. The protein engineering methods described herein can also be applied to other complex proteins.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: E.S.C., T.L., S.P.W., Q.L., N.M., J.L., D.D., A.H., A.I., Zhan Liu, A.V.D.F., A.G., J.S., B.M. and R.P. are current employees of Sanofi and shareholders. M.T. and T.D. are current employees of Biogen and stock holders. J.K., C.F., H.J., Zhiqian Liu, G.F.P., R.M., and T.C. are former employees of Biogen. B.Y., D.R., J.M.S., and O.M. are former employees of Bioverativ, a Sanofi company. V.S. is an employee of Amunix Pharmaceuticals.
Figures


Comment in
-
A molecular jewel for hemophilia A treatment.Blood. 2020 Apr 23;135(17):1417-1419. doi: 10.1182/blood.2020005250. Blood. 2020. PMID: 32324867 No abstract available.
References
-
- Medical and Scientific Advisory Council (MASAC) MASAC document 241: MASAC recommendations concerning prophylaxis. New York: National Hemophilia Foundation. 2016. https://www.hemophilia.org/sites/default/files/document/files/241Prophyl.... Accessed 25 October 2019.
-
- Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. ; Treatment Guidelines Working Group on Behalf of The World Federation Of Hemophilia . Guidelines for the management of hemophilia. Haemophilia. 2013;19(1):e1-e47. - PubMed
-
- Lillicrap D. Improvements in factor concentrates. Curr Opin Hematol. 2010;17(5):393-397. - PubMed
-
- Fogarty PF. Biological rationale for new drugs in the bleeding disorders pipeline. Hematology Am Soc Hematol Educ Program. 2011;2011(1):397-404. - PubMed
-
- Hacker MR, Geraghty S, Manco-Johnson M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia. 2001;7(4):392-396. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

