Applications of Graphene Quantum Dots in Biomedical Sensors
- PMID: 32079119
- PMCID: PMC7070974
- DOI: 10.3390/s20041072
Applications of Graphene Quantum Dots in Biomedical Sensors
Abstract
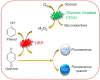
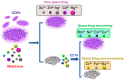
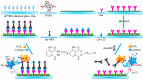
Due to the proliferative cancer rates, cardiovascular diseases, neurodegenerative disorders, autoimmune diseases and a plethora of infections across the globe, it is essential to introduce strategies that can rapidly and specifically detect the ultralow concentrations of relevant biomarkers, pathogens, toxins and pharmaceuticals in biological matrices. Considering these pathophysiologies, various research works have become necessary to fabricate biosensors for their early diagnosis and treatment, using nanomaterials like quantum dots (QDs). These nanomaterials effectively ameliorate the sensor performance with respect to their reproducibility, selectivity as well as sensitivity. In particular, graphene quantum dots (GQDs), which are ideally graphene fragments of nanometer size, constitute discrete features such as acting as attractive fluorophores and excellent electro-catalysts owing to their photo-stability, water-solubility, biocompatibility, non-toxicity and lucrativeness that make them favorable candidates for a wide range of novel biomedical applications. Herein, we reviewed about 300 biomedical studies reported over the last five years which entail the state of art as well as some pioneering ideas with respect to the prominent role of GQDs, especially in the development of optical, electrochemical and photoelectrochemical biosensors. Additionally, we outline the ideal properties of GQDs, their eclectic methods of synthesis, and the general principle behind several biosensing techniques.
Keywords: biomedical applications; biosensors; electrochemical sensors; graphene quantum dots (GQDs); nanomaterials; optical sensors; photoelectrochemical sensors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





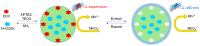

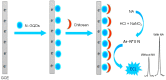
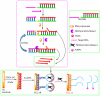






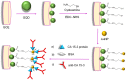


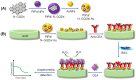





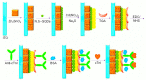
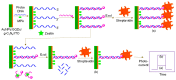


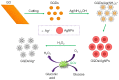
References
-
- Ho K.J. Cardiovascular diseases. Nutr. Asp. Aging. 2018;2:75–100.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

