The Rho/Rac Guanine Nucleotide Exchange Factor Vav1 Regulates Hif-1α and Glut-1 Expression and Glucose Uptake in the Brain
- PMID: 32079227
- PMCID: PMC7072975
- DOI: 10.3390/ijms21041341
The Rho/Rac Guanine Nucleotide Exchange Factor Vav1 Regulates Hif-1α and Glut-1 Expression and Glucose Uptake in the Brain
Abstract
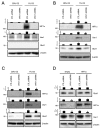
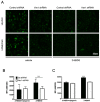
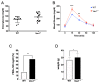
Vav1 is a Rho/Rac (Ras-related C3 botulinum toxin substrate) guanine nucleotide exchange factor expressed in hematopoietic and endothelial cells that are involved in a wide range of cellular functions. It is also stabilized under hypoxic conditions when it regulates the accumulation of the transcription factor HIF (Hypoxia Inducible Factor)-1α, which activates the transcription of target genes to orchestrate a cellular response to low oxygen. One of the genes induced by HIF-1α is GLUT (Glucose Transporter)-1, which is the major glucose transporter expressed in vessels that supply energy to the brain. Here, we identify a role for Vav1 in providing glucose to the brain. We found that Vav1 deficiency downregulates HIF-1α and GLUT-1 levels in endothelial cells, including blood-brain barrier cells. This downregulation of GLUT-1, in turn, reduced glucose uptake to endothelial cells both in vitro and in vivo, and reduced glucose levels in the brain. Furthermore, endothelial cell-specific Vav1 knock-out in mice, which caused glucose uptake deficiency, also led to a learning delay in fear conditioning experiments. Our results suggest that Vav1 promotes learning by activating HIF-1α and GLUT-1 and thereby distributing glucose to the brain. We further demonstrate the importance of glucose transport by endothelial cells in brain functioning and reveal a potential new axis for targeting GLUT-1 deficiency syndromes and other related brain diseases.
Keywords: GLUT-1; HIF-1α; Vav1; hypoxia; learning deficiency.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Huang Y., Lei L., Liu D., Jovin I., Russell R., Johnson R.S., Di Lorenzo A., Giordano F.J. Normal glucose uptake in the brain and heart requires an endothelial cell-specific HIF-1alpha-dependent function. Proc. Natl. Acad. Sci. USA. 2012;109:17478–17483. doi: 10.1073/pnas.1209281109. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

