Fluorescent Analogues of Human α-Calcitonin Gene-Related Peptide with Potent Vasodilator Activity
- PMID: 32079247
- PMCID: PMC7072916
- DOI: 10.3390/ijms21041343
Fluorescent Analogues of Human α-Calcitonin Gene-Related Peptide with Potent Vasodilator Activity
Abstract
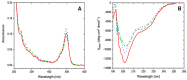
: Human α-calcitonin gene-related peptide (h-α-CGRP) is a highly potent vasodilator peptide that belongs to the family of calcitonin peptides. There are two forms of CGRP receptors in humans and rodents: α-CGRP receptor predominately found in the cardiovascular system and β-CGRP receptor predominating in the gastrointestinal tract. The CGRP receptors are primarily localized to C and Aδ sensory fibers, where they are involved in nociceptive transmission and migraine pathophysiology. These fibers are found both peripherally and centrally, with extensive perivascular location. The CGRP receptors belong to the class B G-protein-coupled receptors, and they are primarily associated to signaling via Gα proteins. The objectives of the present work were: (i) synthesis of three single-labelled fluorescent analogues of h-α-CGRP by 9-fluorenylmethyloxycarbonyl (Fmoc)-based solid-phase peptide synthesis, and (ii) testing of their biological activity in isolated human, mouse, and rat arteries by using a small-vessel myograph setup. The three analogues were labelled with 5(6)-carboxyfluorescein via the spacer 6-aminohexanoic acid at the chain of Lys24 or Lys35. Circular dichroism (CD) experiments were performed to obtain information on the secondary structure of these fluorescently labelled peptides. The CD spectra indicated that the folding of all three analogues was similar to that of native α-CGRP. The three fluorescent analogues of α-CGRP were successfully prepared with a purity of >95%. In comparison to α-CGRP, the three analogues exhibited similar efficacy, but different potency in producing a vasodilator effect. The analogue labelled at the N-terminus proved to be the most readily synthesized, but it was found to possess the lowest vasodilator potency. The analogues labelled at Lys35 or Lys24 exhibited an acceptable reduction in potency (i.e., 3-5 times and 5-10 times less potent, respectively), and thus they have potential for use in further investigations of receptor internalization and neuronal reuptake.
Keywords: 5(6)-carboxyfluorescein; concentration-response curves; human α-calcitonin gene-related peptide; solid-phase peptide synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

