C-H Functionalization via Iron-Catalyzed Carbene-Transfer Reactions
- PMID: 32079259
- PMCID: PMC7070285
- DOI: 10.3390/molecules25040880
C-H Functionalization via Iron-Catalyzed Carbene-Transfer Reactions
Abstract
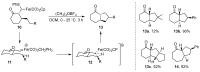
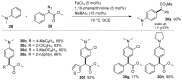
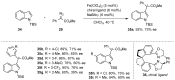
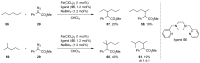
The direct C-H functionalization reaction is one of the most efficient strategies by which to introduce new functional groups into small organic molecules. Over time, iron complexes have emerged as versatile catalysts for carbine-transfer reactions with diazoalkanes under mild and sustainable reaction conditions. In this review, we discuss the advances that have been made using iron catalysts to perform C-H functionalization reactions with diazoalkanes. We give an overview of early examples employing stoichiometric iron carbene complexes and continue with recent advances in the C-H functionalization of C(sp2)-H and C(sp3)-H bonds, concluding with the latest developments in enzymatic C-H functionalization reactions using iron-heme-containing enzymes.
Keywords: C-H functionalization; carbene; diazoalkane; iron.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

















References
-
- Zhang M., Zhang Y., Jie X., Zhao H., Lim G., Su W. Recent advantages in directed C-H functionalizations using monodentate nitrogen-based directing groups. Org. Chem. Front. 2014;1:843–895. doi: 10.1039/C4QO00068D. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

