Effects of Composting Different Types of Organic Fertilizer on the Microbial Community Structure and Antibiotic Resistance Genes
- PMID: 32079314
- PMCID: PMC7074733
- DOI: 10.3390/microorganisms8020268
Effects of Composting Different Types of Organic Fertilizer on the Microbial Community Structure and Antibiotic Resistance Genes
Abstract
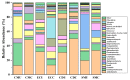
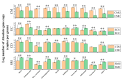
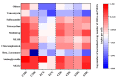
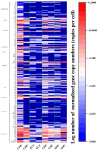
Organic fertilizer is a major carrier that stores and transmits antibiotic resistance genes (ARGs). In the environment, due to the application of organic fertilizers in agriculture, the increasing diversity and abundance of ARGs poses a potential threat to human health and environmental safety. In this paper, the microbial community structure and ARGs in different types of organic fertilizer treated with composting were examined. We found that the abundance and diversity of ARGs in earthworm cast organic fertilizer were the lowest and the highest in chicken manure organic fertilizer. Interestingly, the abundance and diversity of ARGs, especially beta-lactam resistance genes, sulfonamide resistance genes, and macrolide-lincosamide-streptogramin B (MLSB) resistance genes, in organic fertilizers were reduced significantly, while composting caused no significant change in mobile genetic elements (MGEs), where antibiotic deactivation and the use of efflux pumps were the two most dominant mechanisms. It was clear that removal of ARGs became more efficient with increasing reduction in the bacterial abundances and diversity of potential ARG hosts, and integron-mediated horizontal gene transfers (HGTs) played an important role in the proliferation of most ARG types. Therefore, the reduction in ARGs was mainly driven by changes in bacterial community composition caused by composting. Furthermore, rather than HGTs, the diversity and abundance of bacterial communities affected by compost physical and chemical properties were the main drivers shaping and altering the abundance and diversity of ARGs, which was indicated by a correlation analysis of these properties, antibiotic residues, microbial community structure, and ARGs. In general, high-temperature composting effectively removed antibiotic residues and ARGs from these organic fertilizers; however, it cannot prevent the proliferation of MGEs. The insights gained from these results may be of assistance in the safe and rational use of organic fertilizers by indicating the changes in microbial community structure and ARGs in different types of organic fertilizer treated with composting.
Keywords: ARGs; MGEs; antibiotics; composting; microbial community structure; organic fertilizer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Aerobic composting reduces antibiotic resistance genes in cattle manure and the resistome dissemination in agricultural soils.Sci Total Environ. 2018 Jan 15;612:1300-1310. doi: 10.1016/j.scitotenv.2017.09.028. Epub 2017 Sep 8. Sci Total Environ. 2018. PMID: 28898936
-
Fate of tetracycline and sulfonamide resistance genes in a grassland soil amended with different organic fertilizers.Ecotoxicol Environ Saf. 2019 Apr 15;170:39-46. doi: 10.1016/j.ecoenv.2018.11.059. Epub 2018 Dec 1. Ecotoxicol Environ Saf. 2019. PMID: 30513413
-
Microbial organic fertilizer prepared by co-composting of Trichoderma dregs mitigates dissemination of resistance, virulence genes, and bacterial pathogens in soil and rhizosphere.Environ Res. 2024 Jan 15;241:117718. doi: 10.1016/j.envres.2023.117718. Epub 2023 Nov 21. Environ Res. 2024. PMID: 37995998
-
Key factors driving the fate of antibiotic resistance genes and controlling strategies during aerobic composting of animal manure: A review.Sci Total Environ. 2021 Oct 15;791:148372. doi: 10.1016/j.scitotenv.2021.148372. Epub 2021 Jun 8. Sci Total Environ. 2021. PMID: 34139488 Review.
-
Fate of Antibiotic Resistance Genes and Changes in Bacterial Community With Increasing Breeding Scale of Layer Manure.Front Microbiol. 2022 Mar 9;13:857046. doi: 10.3389/fmicb.2022.857046. eCollection 2022. Front Microbiol. 2022. PMID: 35356511 Free PMC article. Review.
Cited by
-
A Shift Pattern of Bacterial Communities Across the Life Stages of the Citrus Red Mite, Panonychus citri.Front Microbiol. 2020 Jul 10;11:1620. doi: 10.3389/fmicb.2020.01620. eCollection 2020. Front Microbiol. 2020. PMID: 32754145 Free PMC article.
-
Fate of Horizontal-Gene-Transfer Markers and Beta-Lactamase Genes during Thermophilic Composting of Human Excreta.Microorganisms. 2023 Jan 24;11(2):308. doi: 10.3390/microorganisms11020308. Microorganisms. 2023. PMID: 36838273 Free PMC article.
-
Impact of Anthropogenic Activities on the Dissemination of ARGs in the Environment-A Review.Int J Environ Res Public Health. 2022 Oct 7;19(19):12853. doi: 10.3390/ijerph191912853. Int J Environ Res Public Health. 2022. PMID: 36232152 Free PMC article. Review.
-
The Evolution of Nutrient and Microbial Composition and Maturity During the Composting of Different Plant-Derived Wastes.Biology (Basel). 2025 Mar 6;14(3):268. doi: 10.3390/biology14030268. Biology (Basel). 2025. PMID: 40136524 Free PMC article.
-
Thermophilic Composting of Human Feces: Development of Bacterial Community Composition and Antimicrobial Resistance Gene Pool.Front Microbiol. 2022 Feb 18;13:824834. doi: 10.3389/fmicb.2022.824834. eCollection 2022. Front Microbiol. 2022. PMID: 35250940 Free PMC article.
References
-
- Harada K., Suzuki M., Kameda M., Mitsuhashi S. On the drug-resistance of enteric bacteria. 2) Transmission of the drug-resistance among Enterobacteriaceae. Jpn. J. Exp. Med. 1960;30:289–299. - PubMed
-
- World Health Organization Overcoming antimicrobial resistance. Am. Heart J. 2000;26:283.
-
- Thiele-Bruhn S. Pharmaceutical antibiotic compounds in soils—A review. J. Plant Nutr. Soil Sci. 2003;166:145–167. doi: 10.1002/jpln.200390023. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

