Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances
- PMID: 32079617
- PMCID: PMC7057416
- DOI: 10.1136/jitc-2019-000433
Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances
Abstract
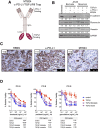
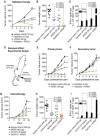
Immunosuppressive entities in the tumor microenvironment (TME) remain a major impediment to immunotherapeutic approaches for a majority of patients with cancer. While the immunosuppressive role of transforming growth factor-β (TGF-β) in the TME is well known, clinical studies to date with anti-TGF-β agents have led to limited success. The bifunctional agent bintrafusp alfa (previously designated M7824) has been developed in an attempt to address this issue. Bintrafusp alfa consists of an IgG1 targeting programmed death ligand 1 (PD-L1) moiety fused via peptide linkers to the extracellular domain of two TGF-β receptor II molecules designed to 'trap' TGF-β in the TME. This agent is able to bring the TGF-β trap to the TME via its anti-PD-L1 component, thus simultaneously attacking both the immunosuppressive PD-L1 and TGF-β entities. A number of preclinical studies have shown bintrafusp alfa capable of (1) preventing or reverting TGF-β-induced epithelial-mesenchymal transition in human carcinoma cells; this alteration in tumor cell plasticity was shown to render human tumor cells more susceptible to immune-mediated attack as well as to several chemotherapeutic agents; (2) altering the phenotype of natural killer and T cells, thus enhancing their cytolytic ability against tumor cells; (3) mediating enhanced lysis of human tumor cells via the antibody-dependent cell-mediated cytotoxicity mechanism; (4) reducing the suppressive activity of Treg cells; (5) mediating antitumor activity in numerous preclinical models and (6) enhancing antitumor activity in combination with radiation, chemotherapy and several other immunotherapeutic agents. A phase I clinical trial demonstrated a safety profile similar to other programmed cell death protein 1 (PD-1)/PD-L1 checkpoint inhibitors, with objective and durable clinical responses. We summarize here preclinical and emerging clinical data in the use of this bispecific and potentially multifunctional agent.
Keywords: TGF-β; checkpoint inhibition; immunotherapy.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
