Predictors of progression in systemic sclerosis patients with interstitial lung disease
- PMID: 32079645
- PMCID: PMC7236865
- DOI: 10.1183/13993003.02026-2019
Predictors of progression in systemic sclerosis patients with interstitial lung disease
Abstract
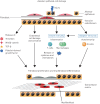
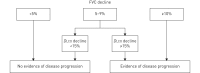
Systemic sclerosis (SSc) is a systemic autoimmune disease affecting multiple organ systems, including the lungs. Interstitial lung disease (ILD) is the leading cause of death in SSc.There are no valid biomarkers to predict the occurrence of SSc-ILD, although auto-antibodies against anti-topoisomerase I and several inflammatory markers are candidate biomarkers that need further evaluation. Chest auscultation, presence of shortness of breath and pulmonary function testing are important diagnostic tools, but lack sensitivity to detect early ILD. Baseline screening with high-resolution computed tomography (HRCT) is therefore necessary to confirm an SSc-ILD diagnosis. Once diagnosed with SSc-ILD, patients' clinical courses are variable and difficult to predict, although certain patient characteristics and biomarkers are associated with disease progression. It is important to monitor patients with SSc-ILD for signs of disease progression, although there is no consensus about which diagnostic tools to use or how often monitoring should occur. In this article, we review methods used to define and predict disease progression in SSc-ILD.There is no valid definition of SSc-ILD disease progression, but we suggest that either a decline in forced vital capacity (FVC) from baseline of ≥10%, or a decline in FVC of 5-9% in association with a decline in diffusing capacity of the lung for carbon monoxide of ≥15% represents progression. An increase in the radiographic extent of ILD on HRCT imaging would also signify progression. A time period of 1-2 years is generally used for this definition, but a decline over a longer time period may also reflect clinically relevant disease progression.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: O. Distler reports personal fees for consultancy from Boehringer, during the conduct of the study; grants and personal fees for lectures from Actelion, personal fees for advisory board work from AbbVie, personal fees for consultancy from Acceleron Pharma, Anamar, Amgen, Catenion, CSL Behring, ChemomAb, Ergonex, GSK, Inventiva, Italfarmaco, iQvia, Medac, Lilly, Sanofi, Target BioScience, UCB, Baecon Discovery, Blade Therapeutics, Curzion Pharmaceuticals and Glenmark Pharmaceuticals, grants and personal fees for consultancy and lectures from Bayer and Boehringer Ingelheim, personal fees for lectures from iQone, Menarini, Mepha and Novartis, personal fees for consultancy and lectures from Medscape, MSD and Roche, grants and personal fees for consultancy from Mitsubishi, personal fees for consultancy and lectures and non-financial (travel) support from Pfizer, outside the submitted work; and has a patent US8247389, EP2331143 issued. Conflict of interest: S. Assassi reports non-financial (writing) support from Boehringer Ingelheim, during the conduct of the study; grants from Biogen Idec and Bayer, grants and personal fees from Boehringer Ingelheim outside the submitted work. Conflict of interest: V. Cottin reports personal fees for advisory board work and lectures and non-financial (travel) support from Actelion, grants, personal fees for advisory board work and lectures, and non-financial (travel) support from Boehringer Ingelheim and Roche, personal fees for advisory board work and data monitoring committee work from Bayer/MSD and Galapagos, personal fees for data monitoring committee work from Gilead, Celgene and Galecto, personal fees for advisory board work and lectures from Novartis, personal fees for lectures from Sanofi, personal fees for steering committee work and data monitoring committee work from Promedior, outside the submitted work. Conflict of interest: M. Cutolo reports grants from Actelion, Boehringer Ingelheim, Horizon, BMS and Celgene, outside the submitted work. Conflict of interest: S.K. Danoff reports grants and personal fees from Boehringer Ingelheim, grants from Genentech/Roche and Bristol Myers Squibb, personal fees from Galapagos and Galectic, during the conduct of the study. Conflict of interest: C.P. Denton reports personal fees from Actelion, Bayer, Sanofi, Boehringer Ingelheim and Corbus, grants and personal fees from GlaxoSmithKline, Inventiva, CSL Behring and Leadiant Biosciences, during the conduct of the study. Conflict of interest: J.H.W. Distler reports personal fees for speaking and advisory board work from Boehringer Ingelheim. Conflict of interest: A-M. Hoffmann-Vold reports non-financial support from Boehringer Ingelheim, during the conduct of the study; grants, personal fees and non-financial support from Boehringer Ingelheim, personal fees and non-financial support from Actelion, personal fees from Roche, outside the submitted work. Conflict of interest: S.R. Johnson reports grants from Boehringer Ingelheim, Corbus, Bayer, Roche and the Canadian Institutes of Health Research outside the submitted work. Conflict of interest: U. Müller Ladner reports personal fees for advisory board work and lectures from Boehringer Ingelheim, outside the submitted work. Conflict of interest: V. Smith reports speaker fees from Boehringer Ingelheim, during the conduct of the study; grants from Boehringer Ingelheim, research funding from Actelion Pharmaceuticals Ltd, Bayer AG, Hoffman-La Roche AG, Galapagos NV and Sanofi, outside the submitted work. Conflict of interest: E.R. Volkmann reports personal fees from Boehringer Ingelheim, outside the submitted work. Conflict of interest: T.M. Maher reports grants and personal fees from GSK and AstraZeneca, grants, personal fees and non-financial support from UCB, personal fees from Boehringer Ingelheim, Roche, Bayer, Prometic, Samumed, Galapagos, Celgene, Indalo, Pliant, Blade Therapeutics, Respivant, Novartis and Bristol-Myers Squibb, stock options from Apellis, outside the submitted work.
Figures

References
-
- Varga J, Trojanowska M, Kuwana M. Pathogenesis of systemic sclerosis: recent insights of molecular and cellular mechanisms and therapeutic opportunities. J Scleroderma Relat Disord 2017; 2: 137–152. doi: 10.5301/jsrd.5000249 - DOI
