PTPN23 binds the dynein adaptor BICD1 and is required for endocytic sorting of neurotrophin receptors
- PMID: 32079660
- PMCID: PMC7132798
- DOI: 10.1242/jcs.242412
PTPN23 binds the dynein adaptor BICD1 and is required for endocytic sorting of neurotrophin receptors
Abstract
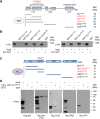
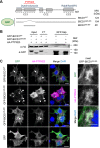
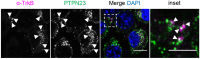
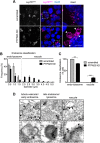
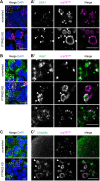
Signalling by target-derived neurotrophins is essential for the correct development of the nervous system and its maintenance throughout life. Several aspects concerning the lifecycle of neurotrophins and their receptors have been characterised over the years, including the formation, endocytosis and trafficking of signalling-competent ligand-receptor complexes. However, the molecular mechanisms directing the sorting of activated neurotrophin receptors are still elusive. Previously, our laboratory identified Bicaudal-D1 (BICD1), a dynein motor adaptor, as a key factor for lysosomal degradation of brain-derived neurotrophic factor (BDNF)-activated TrkB (also known as NTRK2) and p75NTR (also known as NGFR) in motor neurons. Here, using a proteomics approach, we identified protein tyrosine phosphatase, non-receptor type 23 (PTPN23), a member of the endosomal sorting complexes required for transport (ESCRT) machinery, in the BICD1 interactome. Molecular mapping revealed that PTPN23 is not a canonical BICD1 cargo; instead, PTPN23 binds the N-terminus of BICD1, which is also essential for the recruitment of cytoplasmic dynein. In line with the BICD1-knockdown phenotype, loss of PTPN23 leads to increased accumulation of BDNF-activated p75NTR and TrkB in swollen vacuole-like compartments, suggesting that neuronal PTPN23 is a novel regulator of the endocytic sorting of neurotrophin receptors.
Keywords: Intracellular sorting; Motor neuron; Trafficking; TrkB; p75NTR.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


References
-
- Alazami A. M., Patel N., Shamseldin H. E., Anazi S., Al-Dosari M. S., Alzahrani F., Hijazi H., Alshammari M., Aldahmesh M. A., Salih M. A. et al. (2015). Accelerating novel candidate gene discovery in neurogenetic disorders via whole-exome sequencing of prescreened multiplex consanguineous families. Cell Rep. 10, 148-161. 10.1016/j.celrep.2014.12.015 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- SCHIAVO/OCT15/880-792/MNDA_/Motor Neurone Disease Association/United Kingdom
- 515092 /BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 107116/Z/15/Z /WT_/Wellcome Trust/United Kingdom
- 533334 /BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

