Iron deficiency-induced thrombocytosis increases thrombotic tendency in rats
- PMID: 32079699
- PMCID: PMC7928018
- DOI: 10.3324/haematol.2019.245092
Iron deficiency-induced thrombocytosis increases thrombotic tendency in rats
Abstract
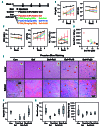
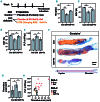
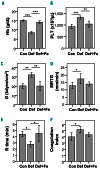
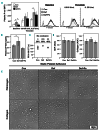
Iron deficiency (ID) is globally prevalent, and apart from anemia is associated with thrombocytosis. While considered benign, studies linking thrombotic events with prior ID anemia suggest otherwise. Herein we used animal models to assess the influence of ID on thrombotic tendency. Sprague-Dawley rats were fed control or iron deficient diets and ferric carboxymaltose was used to reverse ID. Thrombosis was induced via stenosis of the inferior vena cava or damage to the right carotid artery using ferric chloride. Thrombi were evaluated histologically and via high frequency ultrasound in the venous model. ID consistently induced thrombocytosis alongside anemia. Venous thrombus growth and final dimensions in both arterial and venous thrombi were largest in ID. In both models, platelet numbers correlated with the final thrombus size, with ID thrombi having the largest platelet areas. Platelet function was also evaluated in surgically naive rats. Coagulability on thromboelastography and hemostasis on tail transection were augmented in ID. Platelet and plasma P-selectin expression were both higher in ID. Platelet adhesion and aggregation in ID was impaired under shear flow but was intact on static assays. Iron replacement therapy reversed all ID-related changes in hematological parameters, thrombus dimensions, and platelet assays. In summary, ID alone increases thrombotic tendency. Iron replacement therapy reverses these changes, making it a viable strategy for prevention of ID-related thrombotic disease. This may be of importance in patients with chronic illnesses which may already be at increased risk for thrombosis such as inflammatory bowel disease, chronic kidney disease, or cancer.
Figures



Comment in
-
Recognition of thrombotic risk of thrombocytosis in iron deficiency.Haematologica. 2021 Mar 1;106(3):661-663. doi: 10.3324/haematol.2020.270496. Haematologica. 2021. PMID: 33645945 Free PMC article. No abstract available.
References
-
- Camaschella C. Iron deficiency. Blood. 2019;133(1):30-39. - PubMed
-
- Evstatiev R, Gasche C. Iron sensing and signalling. Gut. 2012;61(6):933-952. - PubMed
-
- von Haehling S, Jankowska EA, van Veldhuisen DJ, Ponikowski P, Anker SD. Iron deficiency and cardiovascular disease. Nat Rev Cardiol. 2015;12(11):659-669. - PubMed
-
- Dan K. Thrombocytosis in iron deficiency anemia. Intern Med. 2005;44(10):1025-1026. - PubMed
-
- Keung YK, Owen J. Iron deficiency and thrombosis: literature review. Clin Appl Thromb Hemost. 2004;10(4):387-391. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

