Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of B-cell lymphomas
- PMID: 32079701
- PMCID: PMC7849551
- DOI: 10.3324/haematol.2019.241208
Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of B-cell lymphomas
Abstract
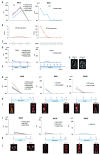
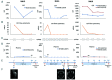
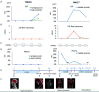
The levels of cell free circulating tumor DNA (ctDNA) in plasma correlated with treatment response and outcome in systemic lymphomas. Notably, in brain tumors, the levels of ctDNA in the cerebrospinal fluid (CSF) are higher than in plasma. Nevertheless, their role in central nervous system (CNS) lymphomas remains elusive. We evaluated the CSF and plasma from 19 patients: 6 restricted CNS lymphomas, 1 systemic and CNS lymphoma, and 12 systemic lymphomas. We performed whole exome sequencing or targeted sequencing to identify somatic mutations of the primary tumor, then variant-specific droplet digital PCR was designed for each mutation. At time of enrolment, we found ctDNA in the CSF of all patients with restricted CNS lymphoma but not in patients with systemic lymphoma without CNS involvement. Conversely, plasma ctDNA was detected in only 2/6 patients with restricted CNS lymphoma with lower variant allele frequencies than CSF ctDNA. Moreover, we detected CSF ctDNA in 1 patient with CNS lymphoma in complete remission and in 1 patient with systemic lymphoma, 3 and 8 months before CNS relapse was confirmed; indicating CSF ctDNA might detect CNS relapse earlier than conventional methods. Finally, in 2 cases with CNS lymphoma, CSF ctDNA was still detected after treatment even though a complete decrease in CSF tumor cells was observed by flow cytometry (FC), indicating CSF ctDNA better detected residual disease than FC. In conclusion, CSF ctDNA can better detect CNS lesions than plasma ctDNA and FC. In addition, CSF ctDNA predicted CNS relapse in CNS and systemic lymphomas.
Figures

References
-
- Yamamoto W, Tomita N, Watanabe R, et al. Central nervous system involvement in diffuse large B-cell lymphoma. Eur J Haematol. 2010;85(1):6-10. - PubMed
-
- WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. Lyon, France: IARC; 2017.
-
- Quijano S, López A, Manuel Sancho J, et al. Identification of leptomeningeal disease in aggressive B-cell non-Hodgkin's lymphoma: improved sensitivity of flow cytometry. J Clin Oncol. 2009;27(9):1462-1469. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

