Unrestricted use of polymer-free sirolimus eluting stents in routine clinical practice
- PMID: 32080086
- PMCID: PMC7034709
- DOI: 10.1097/MD.0000000000019119
Unrestricted use of polymer-free sirolimus eluting stents in routine clinical practice
Abstract
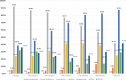
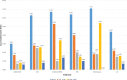
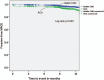
Stent designs with ultrathin struts may further increase the procedural success of challenging lesion subsets. The objective of this study was to assess the safety and efficacy of ultrathin strut, polymer-free sirolimus eluting stent (PF-SES) implantations in a large scale, unselected patient population.Adult patients underwent percutaneous coronary interventions (PCI) with a thin-strut PF-SES. Data from two all-comers observational studies having the same protocol (ClinicalTrials.gov Identifiers: NCT02629575 and NCT02905214) were pooled. The accumulated target lesion revascularization (TLR) rate at 9-12 months was the primary endpoint. All dual antiplatelet therapy strategies according to the applicable guidelines were permissible.In total, 7243 patients were prospectively enrolled for PCI with PF-SES in stable coronary artery disease or acute coronary syndrome (ACS). Major risk factors in the overall cohort were diabetes (37.3%), ST elevation myocardial infarction (18.1%) and non-ST myocardial infarction (24.6%). The follow-up rate was 88.6% in the overall population. The TLR rate in the overall cohort was 2.2% whereas definite/probable stent thrombosis (ST) occurred in 0.7%. In patients with in-stent restenosis lesions, the major adverse cardiac events rate was 6.4% whereas the corresponding rate for isolated left main coronary artery (LMCA) disease was highest with 6.7% followed by patients with culprit lesions in vein bypasses (VB, 7.1%). The mortality rate in patients treated in VB lesions was highest with 5.4%, followed by the isolated LMCA subgroup (3.4%) and ACS (2.6%).PCI with PF-SES in an unselected patient population, is associated with low clinical event and ST rates. Furthermore, PF-SES angioplasty in niche indications demonstrated favorable safety and efficacy outcomes with high procedural success rates.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Yamaji K, Zanchin T, Zanchin C, et al. Unselected use of ultrathin strut biodegradable polymer sirolimus-eluting stent versus durable polymer everolimus-eluting stent for coronary revascularization. Circ Cardiovasc Interv 2018;11:e006741. - PubMed
-
- Maeng M, Christiansen EH, Raungaard B, et al. SORT OUT VIII investigators. Everolimus-eluting versus biolimus-eluting stents with biodegradable polymers in unselected patients undergoing percutaneous coronary intervention: a randomized noninferiority trial with 1-year follow-up (SORT OUT VIII Trial). JACC Cardiovasc Interv 2019;12:624–33. - PubMed
-
- Krackhardt F, Rosli MA, Leschke M, et al. Propensity score matched all comers population treated with ultra-thin strut bare metal and sirolimus-probucol coated drug-eluting stents of identical stent architecture. Catheter Cardiovasc Interv 2018;91:1221–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

