A pilot study on efficacy and safety of a new salt substitute with very low sodium among hypertension patients on regular treatment
- PMID: 32080135
- PMCID: PMC7034699
- DOI: 10.1097/MD.0000000000019263
A pilot study on efficacy and safety of a new salt substitute with very low sodium among hypertension patients on regular treatment
Abstract
Objectives: To understand the possible effect of a novel salt substitute with very low sodium in reducing blood pressure, salt intake and use of anti-hypertensive medications among patients on regular medications, to inform the future randomized trials.
Design: Single-arm pilot trial.
Setting: A community health service center in Chongqing, China.
Participants: A total of 43 patients with hypertension taking anti-hypertensive medications regularly.
Intervention: Patients received the salt substitute with 18% sodium chloride for 8 weeks.
Main outcome measures: Patients were followed up weekly for the use of antihypertensive medications and measurements of blood pressure. We collected 24-h urine before and after the trial to measure sodium and potassium intake.
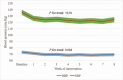
Results: Among 39 patients who completed the 8 weeks' intervention, 30.8% patients stopped or reduced anti-hypertensive medications during the trial. For patients that stopped or reduced medication, the mean SBP and DBP before intervention were 122.1 ± 9.6 and 68.9 ± 9.4 mm Hg and both did not increase after intervention (SBP change: 2.8 mm Hg (-5.1, 10.8), P = .48; DBP change: 1.8 mm Hg (-2.2, 5.7), P = .38). For the rest patients, the mean SBP and DBP before intervention were 141.6 ± 16.9 and 74.6 ± 6.6 mm Hg but reduced significantly after the intervention (SBP change: -16.0 mm Hg (-21.3, -10.6), P < .001; DBP change: -5.5 mm Hg (-8.1, -2.9), P < .001). The 24-h urine sodium decreased (P < .001) and potassium increased (P < .001) among all patients. No severe adverse events were reported.
Conclusions: The novel salt substitute showed potential in reducing blood pressure and use of antihypertensive medications. Further randomized double-blind controlled trial is warranted to validate these findings.Clinical Trial Registration-URL:http://www.clinicaltrials.gov. Unique identifier: NCT03226327.
Figures
References
-
- Lawes CM, Hoorn SV, Rodgers A. Global burden of blood–pressure–related disease. Lancet 2001;371:1513–8. - PubMed
-
- Asaria P, Chisholm D, Mathers C, et al. Chronic disease prevention: health effects and financial costs of strategies to reduce salt intake and control tobacco use. Lancet 2007;370:2044–53. - PubMed
-
- Yuan P, Zhang SF. Overview of excessive salt intake. Henan J Prev Med 2013;24:268–70.
-
- Chen X, Ma JX, Guo XL, et al. Domestic and foreign strategies and actions for reducing salt and preventing hypertension. Prev Med Tribune 2011;17:817–21.
-
- Hu JH, Zhao LC, Li X. Effects of salt substitution on blood pressure using home measurements in essential hypertensive patients: a double–blinded randomized controlled trial. Chin J Hypertens 2014;22:42–6.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

