Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species
- PMID: 32080228
- PMCID: PMC7033188
- DOI: 10.1038/s41598-020-59652-6
Antibacterial effects of low-temperature plasma generated by atmospheric-pressure plasma jet are mediated by reactive oxygen species
Abstract
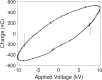
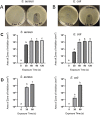
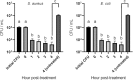
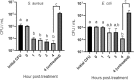
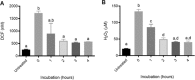
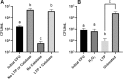
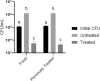
Emergence and spread of antibiotic resistance calls for development of non-chemical treatment options for bacterial infections. Plasma medicine applies low-temperature plasma (LTP) physics to address biomedical problems such as wound healing and tumor suppression. LTP has also been used for surface disinfection. However, there is still much to be learned regarding the effectiveness of LTP on bacteria in suspension in liquids, and especially on porous surfaces. We investigated the efficacy of LTP treatments against bacteria using an atmospheric-pressure plasma jet and show that LTP treatments have the ability to inhibit both gram-positive (S. aureus) and gram-negative (E. coli) bacteria on solid and porous surfaces. Additionally, both direct LTP treatment and plasma-activated media were effective against the bacteria suspended in liquid culture. Our data indicate that reactive oxygen species are the key mediators of the bactericidal effects of LTP and hydrogen peroxide is necessary but not sufficient for antibacterial effects. In addition, our data suggests that bacteria exposed to LTP do not develop resistance to further treatment with LTP. These findings suggest that this novel atmospheric-pressure plasma jet could be used as a potential alternative to antibiotic treatments in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Centers for Disease Control and Prevention. About Antimicrobial Resistance | Antibiotic/Antimicrobial Resistance | CDC. Available at, https://www.cdc.gov/drugresistance/about.html. (Accessed: 19th December 2018) (2018).
-
- World Health Organization. WHO Library Cataloguing-in-Publication Data Global Action Plan on Antimicrobial Resistance (2015).
-
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report Early implementation. (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

