Conditional Disorder in Small Heat-shock Proteins
- PMID: 32081587
- PMCID: PMC7245567
- DOI: 10.1016/j.jmb.2020.02.003
Conditional Disorder in Small Heat-shock Proteins
Abstract
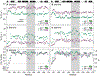
Small heat-shock proteins (sHSPs) are molecular chaperones that respond to cellular stresses to combat protein aggregation. HSP27 is a critical human sHSP that forms large, dynamic oligomers whose quaternary structures and chaperone activities depend on environmental factors. Upon exposure to cellular stresses, such as heat shock or acidosis, HSP27 oligomers can dissociate into dimers and monomers, which leads to significantly enhanced chaperone activity. The structured core of the protein, the α-crystallin domain (ACD), forms dimers and can prevent the aggregation of substrate proteins to a similar degree as the full-length protein. When the ACD dimer dissociates into monomers, it partially unfolds and exhibits enhanced activity. Here, we used solution-state NMR spectroscopy to characterize the structure and dynamics of the HSP27 ACD monomer. Web show that the monomer is stabilized at low pH and that its backbone chemical shifts, 15N relaxation rates, and 1H-15N residual dipolar couplings suggest structural changes and rapid motions in the region responsible for dimerization. By analyzing the solvent accessible and buried surface areas of sHSP structures in the context of a database of dimers that are known to dissociate into disordered monomers, we predict that ACD dimers from sHSPs across all kingdoms of life may partially unfold upon dissociation. We propose a general model in which conditional disorder-the partial unfolding of ACDs upon monomerization-is a common mechanism for sHSP activity.
Keywords: Conditional disorder; Molecular chaperone; NMR; Residual dipolar couplings; Small heat-shock protein.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures



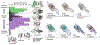
References
-
- Kampinga HH, de Boer R, Beerstra N, The multicolored world of the human HSPB family, Springer International Publishing, Cham, 2015. doi:10.1007/978-3-319-16077-1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- BB/J014346/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J018082/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- Z01 DK029046/ImNIH/Intramural NIH HHS/United States
- P41 GM111135/GM/NIGMS NIH HHS/United States
- Z01 DK029048/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

