Discovery and development of safe-in-man broad-spectrum antiviral agents
- PMID: 32081774
- PMCID: PMC7128205
- DOI: 10.1016/j.ijid.2020.02.018
Discovery and development of safe-in-man broad-spectrum antiviral agents
Abstract
Viral diseases are one of the leading causes of morbidity and mortality in the world. Virus-specific vaccines and antiviral drugs are the most powerful tools to combat viral diseases. However, broad-spectrum antiviral agents (BSAAs, i.e. compounds targeting viruses belonging to two or more viral families) could provide additional protection of the general population from emerging and re-emerging viral diseases, reinforcing the arsenal of available antiviral options. Here, we review discovery and development of BSAAs and summarize the information on 120 safe-in-man agents in a freely accessible database (https://drugvirus.info/). Future and ongoing pre-clinical and clinical studies will increase the number of BSAAs, expand the spectrum of their indications, and identify drug combinations for treatment of emerging and re-emerging viral infections as well as co-infections.
Keywords: Antiviral drug; BSAAs; Broad-spectrum antiviral agents; Drug discovery and development; Virus.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

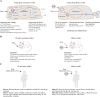
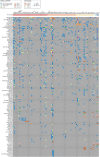

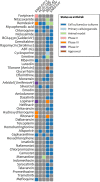
References
-
- Alabaster V., In Vivo Pharmacology Training Group The fall and rise of in vivo pharmacology. Trends Pharmacol Sci. 2002;23:13–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

