Sex-dependent effects of chronic variable stress on discrete corticotropin-releasing factor receptor 1 cell populations
- PMID: 32081812
- PMCID: PMC7540729
- DOI: 10.1016/j.physbeh.2020.112847
Sex-dependent effects of chronic variable stress on discrete corticotropin-releasing factor receptor 1 cell populations
Abstract
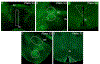
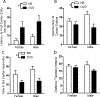
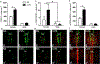
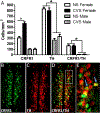
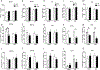
Anxiety and depression are strikingly more prevalent in women compared with men. Dysregulation of corticotropin-releasing factor (CRF) binding to its cognate receptor (CRFR1) is thought to play a critical role in the etiology of these disorders. In the present study, we investigated whether there were sex differences in the effects of chronic variable stress (CVS) on CRFR1 cells using CRFR1-GFP reporter mice experiencing a 9-day CVS paradigm. Brains were collected from CVS and stress naïve female and male mice following exposure to the open field test. This CVS paradigm effectively increased anxiety-like behavior in female and male mice. In addition, we assessed changes in activation of CRFR1 cells (co-localization with c-Fos and phosphorylated CREB (pCREB)) in stress associated brain structures, including two sexually dimorphic CRFR1 cell groups in the anteroventral periventricular nucleus (AVPV/PeN; F>M) and paraventricular hypothalamus (PVN; M>F). CVS increased CRFR1-GFP cell number as well as the number of CRFR1/pCREB co-expressing cells in the female but not male AVPV/PeN. In the PVN, the number of CRFR1/pCREB co-expressing cells was overall greater in males regardless of treatment and CVS resulted in a male-specific reduction of CRFR1/c-Fos cells. In addition, CVS induced a female-specific reduction in CRFR1/c-Fos cells within the anteroventral bed nucleus of the stria terminalis and both sexes exhibited a reduction in CRFR1/c-Fos co-expressing cells following CVS within the ventral basolateral amygdala. Overall, these sex-specific effects of CVS on CRFR1 populations may have implications for sex differences in stress-induction of mood disorders.
Keywords: Anxiety; Chronic stress; Corticotropin releasing factor; Sex difference.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures


References
-
- Aguilera G (1994), Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendo 15:321–350. - PubMed
-
- Anisman H, Prakash P, Merali Z, Poulter MO (2007), Corticotropin releasing hormone receptor alterations elicited by acute and chronic unpredictable stressor challenges in stressor-susceptible and resilient strains of mice. Behav Brain Res 181(2):180–90. - PubMed
-
- Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice (2009). In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. 2nd edition. Boca Raton (FL): CRC Press/Taylor & Francis; Chapter 5.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

