Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior
- PMID: 32081848
- PMCID: PMC7035248
- DOI: 10.1038/s41467-020-14740-z
Prefrontal parvalbumin interneurons require juvenile social experience to establish adult social behavior
Abstract
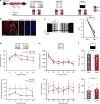
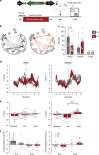
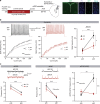
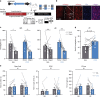
Social isolation during the juvenile critical window is detrimental to proper functioning of the prefrontal cortex (PFC) and establishment of appropriate adult social behaviors. However, the specific circuits that undergo social experience-dependent maturation to regulate social behavior are poorly understood. We identify a specific activation pattern of parvalbumin-positive interneurons (PVIs) in dorsal-medial PFC (dmPFC) prior to an active bout, or a bout initiated by the focal mouse, but not during a passive bout when mice are explored by a stimulus mouse. Optogenetic and chemogenetic manipulation reveals that brief dmPFC-PVI activation triggers an active social approach to promote sociability. Juvenile social isolation decouples dmPFC-PVI activation from subsequent active social approach by freezing the functional maturation process of dmPFC-PVIs during the juvenile-to-adult transition. Chemogenetic activation of dmPFC-PVI activity in the adult animal mitigates juvenile isolation-induced social deficits. Therefore, social experience-dependent maturation of dmPFC-PVI is linked to long-term impacts on social behavior.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Yamamuro Kazuhiko, Yoshino Hiroki, Ogawa Yoichi, Makinodan Manabu, Toritsuka Michihiro, Yamashita Masayuki, Corfas Gabriel, Kishimoto Toshifumi. Social Isolation During the Critical Period Reduces Synaptic and Intrinsic Excitability of a Subtype of Pyramidal Cell in Mouse Prefrontal Cortex. Cerebral Cortex. 2017;28(3):998–1010. doi: 10.1093/cercor/bhx010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

