Effects of SDS on the activity and conformation of protein tyrosine phosphatase from thermus thermophilus HB27
- PMID: 32081966
- PMCID: PMC7035334
- DOI: 10.1038/s41598-020-60263-4
Effects of SDS on the activity and conformation of protein tyrosine phosphatase from thermus thermophilus HB27
Abstract
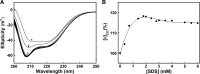
Deciphering the activity-conformation relationship of PTPase is of great interest to understand how PTPase activity is determined by its conformation. Here we studied the activity and conformational transitions of PTPase from thermus thermophilus HB27 in the presence of sodium dodecyl sulfate (SDS). Activity assays showed the inactivation of PTPase induced by SDS was in a concentration-dependent manner. Fluorescence and circular dichroism spectra suggested SDS induced significant conformational transitions of PTPase, which resulted in the inactivation of PTPase, and the changes of α-helical structure and tertiary structure of PTPase. Structural analysis revealed a number of hydrophobic and charged residues around the active sites of PTPase may be involved in the hydrophobic and ionic bonds interactions of PTPase and SDS, which are suggested to be the major driving force to result in PTPase inactivation and conformational transitions induced by SDS. Our results suggested the hydrophobic and charged residues around the active sites were essential for the activity and conformation of PTPase. Our study promotes a better understanding of the activity and conformation of PTPase.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Inactivation and unfolding of protein tyrosine phosphatase from Thermus thermophilus HB27 during urea and guanidine hydrochloride denaturation.PLoS One. 2014 Sep 25;9(9):e107932. doi: 10.1371/journal.pone.0107932. eCollection 2014. PLoS One. 2014. PMID: 25255086 Free PMC article.
-
Expression, purification and characterization of recombinant protein tyrosine phosphatase from Thermus thermophilus HB27.Acta Biochim Biophys Sin (Shanghai). 2009 Aug;41(8):689-98. doi: 10.1093/abbs/gmp057. Acta Biochim Biophys Sin (Shanghai). 2009. PMID: 19657570
-
Inactivation and unfolding of the hyperthermophilic inorganic pyrophosphatase from Thermus thermophilus by sodium dodecyl sulfate.Int J Mol Sci. 2009 Jun 23;10(6):2849-2859. doi: 10.3390/ijms10062849. Int J Mol Sci. 2009. PMID: 19582233 Free PMC article.
-
A temperature-dependent conformational change of NADH oxidase from Thermus thermophilus HB8.Proteins. 2012 Feb;80(2):546-55. doi: 10.1002/prot.23219. Epub 2011 Nov 12. Proteins. 2012. PMID: 22081476 Free PMC article.
-
pH-dependent differential interacting mechanisms of sodium dodecyl sulfate with bovine serum fetuin: a biophysical insight.J Phys Chem B. 2014 Nov 20;118(46):13025-36. doi: 10.1021/jp501515g. Epub 2014 Nov 7. J Phys Chem B. 2014. PMID: 25338219
Cited by
-
Biophysical characteristics of lipid-induced Aβ oligomers correlate to distinctive phenotypes in transgenic mice.FASEB J. 2021 Feb;35(2):e21318. doi: 10.1096/fj.202002025RR. FASEB J. 2021. PMID: 33508158 Free PMC article.
-
A psychrotolerant extracellular phosphatase from Krossfjorden sediment bacterium Bacillus cereus KR_O9: purification and functional characterization.World J Microbiol Biotechnol. 2025 Feb 14;41(2):72. doi: 10.1007/s11274-025-04252-7. World J Microbiol Biotechnol. 2025. PMID: 39948259
-
Trimer stability of Helicobacter pylori HtrA is regulated by a natural mutation in the protease domain.Med Microbiol Immunol. 2023 Jun;212(3):241-252. doi: 10.1007/s00430-023-00766-9. Epub 2023 May 14. Med Microbiol Immunol. 2023. PMID: 37183214 Free PMC article.
-
First Phosphonic-Type Inhibitors and Activity-Based Probes Specific to the O'nyong-nyong Virus Capsid Protease.ChemMedChem. 2025 Jun 17;20(12):e202500049. doi: 10.1002/cmdc.202500049. Epub 2025 Apr 18. ChemMedChem. 2025. PMID: 40192485 Free PMC article.
-
Efficient Expression and Characterization of an Endo-Type Lyase HCLase_M28 and Its Gradual Scale-Up Fermentation for the Preparation of Chondroitin Sulfate Oligosaccharides.Appl Biochem Biotechnol. 2024 Sep;196(9):6526-6555. doi: 10.1007/s12010-024-04878-7. Epub 2024 Feb 22. Appl Biochem Biotechnol. 2024. PMID: 38386140
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

