Montelukast, an Anti-asthmatic Drug, Inhibits Zika Virus Infection by Disrupting Viral Integrity
- PMID: 32082265
- PMCID: PMC7002393
- DOI: 10.3389/fmicb.2019.03079
Montelukast, an Anti-asthmatic Drug, Inhibits Zika Virus Infection by Disrupting Viral Integrity
Abstract
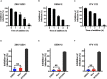
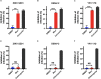
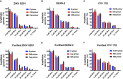
The association of Zika virus (ZIKV) infection and severe complications including neurological sequelae especially fetal microcephaly has aroused global attentions since its outbreak in 2015. Currently, there are no vaccines or therapeutic drugs clinically approved for treatments of ZIKV infection, however. And the drugs used for treating ZIKV in pregnant women require a higher safety profile. Here, we identified an anti-asthmatic drug, montelukast, which is of safety profile for pregnant women and exhibited antiviral efficacy against ZIKV infection in vitro and in vivo. And we showed that montelukast could disrupt the integrity of the virions to release the viral genomic RNA, hence irreversibly inhibiting viral infectivity. In consideration of the neuro-protective activity that montelukast possessed, which was previously reported, it is promising that montelukast could be used for patients with ZIKV infection, particularly for pregnant women.
Keywords: Zika virus; flavivirus; montelukast; viral inactivator; viral integrity.
Copyright © 2020 Chen, Li, Wang and Zou.
Figures






References
-
- Ahmad A., Waseem T., Butt N., Randhawa F., Malik U., Shakoori T. (2018). Montelukast reduces the risk of dengue shock syndrome in dengue patients. Trop. Biomed. 35 1115–1122. - PubMed
LinkOut - more resources
Full Text Sources

