Insights Into the Role of Endoplasmic Reticulum Stress in Infectious Diseases
- PMID: 32082307
- PMCID: PMC7005066
- DOI: 10.3389/fimmu.2019.03147
Insights Into the Role of Endoplasmic Reticulum Stress in Infectious Diseases
Abstract
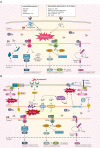
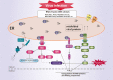
The endoplasmic reticulum (ER) is the major organelle in the cell for protein folding and plays an important role in cellular functions. The unfolded protein response (UPR) is activated in response to misfolded or unfolded protein accumulation in the ER. However, the UPR successfully alleviates the ER stress. If UPR fails to restore ER homeostasis, apoptosis is induced. ER stress plays an important role in innate immune signaling in response to microorganisms. Dysregulation of UPR signaling contributes to the pathogenesis of a variety of infectious diseases. In this review, we summarize the contribution of ER stress to the innate immune response to invading microorganisms and its role in the pathogenesis of infectious diseases.
Keywords: ER stress; UPR (unfolded protein response); apoptosis; bacteria; infection; infectious disease; pathogen; viruses.
Copyright © 2020 Choi and Song.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

