Event-Driven Immunoprofiling Predicts Return of Disease Activity in Alemtuzumab-Treated Multiple Sclerosis
- PMID: 32082320
- PMCID: PMC7005935
- DOI: 10.3389/fimmu.2020.00056
Event-Driven Immunoprofiling Predicts Return of Disease Activity in Alemtuzumab-Treated Multiple Sclerosis
Abstract
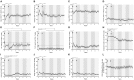
Background: Alemtuzumab is a highly effective drug for the treatment of multiple sclerosis (MS), characterized by specific patterns of depletion and repopulation. As an induction-like treatment concept, two mandatory infusion courses can inhibit long-term disease activity in the majority of patients, and additional courses can successfully manage subsequent re-emergence of disease activity. Currently, there are no biomarkers to identify patients with re-emergent disease activity requiring retreatment. Methods: In this study, we systematically characterized 16 MS patients commencing alemtuzumab. Clinical parameters, MRI and detailed immunoprofiling were conducted every 3 months for up to 84 months. Results: Alemtuzumab led to significant decrease in clinical disease activity in all evaluated patients. Nine out of 16 patients presented with no evidence of disease activity (NEDA)-3 up to 84 months ("complete-responder"), while 7 patients demonstrated clinical or/and subclinical MRI disease activity and received alemutzumab retreatment ("partial-responder"). In both response categories, all T- and B-cell subsets were markedly depleted after alemtuzumab therapy. In particular, absolute numbers of Th1 and Th17 cells were markedly decreased and remained stable below baseline levels-this effect was particularly pronounced in complete-responders. While mean cell numbers did not differ significantly between groups, analysis of event-driven immunoprofiling demonstrated that absolute numbers of Th1 and Th17 cells showed a reproducible increase starting 6 months before relapse activity. This change appears to predict emergent disease activity when compared with stable disease. Conclusion: Studies with larger patient populations are needed to confirm that frequent immunoprofiling may assist in evaluating clinical decision-making of alemtuzumab retreatment.
Keywords: alemtuzumab; immune cells; immunoprofiling; multiple sclerosis; treatment response.
Copyright © 2020 Akgün, Blankenburg, Marggraf, Haase and Ziemssen.
Figures





References
-
- Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. . Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. (2012) 380:1819–28. 10.1016/S0140-6736(12)61769-3 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

