Effect of Different Exercise Loads on Testicular Oxidative Stress and Reproductive Function in Obese Male Mice
- PMID: 32082477
- PMCID: PMC7007943
- DOI: 10.1155/2020/3071658
Effect of Different Exercise Loads on Testicular Oxidative Stress and Reproductive Function in Obese Male Mice
Abstract
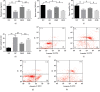
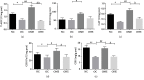
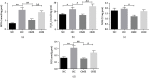
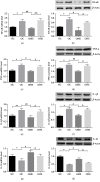
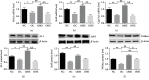
This study is aimed at investigating the effect of different exercise loads on the reproductive function of obese male mice and the underlying mechanisms. Male mice with high-fat diet-induced obesity were divided into obesity control (OC), obesity moderate-load exercise (OME), and obesity high-load exercise (OHE) groups. The OME and OHE groups were subjected to swimming exercise 5 days per week over a duration of 8 weeks, with the exercise load progressively increased to 2 h per day in the OME group and 2 h twice per day in the OHE group. In the OC group mice without exercise regimen, we observed a decrease in mRNA expression of antioxidant enzymes, increase in free radical products, upregulation of mRNA and protein expression of nuclear factor-κB and proinflammatory cytokines, inhibition of mRNA and protein expression of testosterone synthases, decrease in the serum testosterone level and sperm quality, and increase in sperm apoptosis. Although both moderate-load exercise and high-load exercise reduced body fat, only moderate-load exercise effectively alleviated obesity-induced oxidative stress, downregulated the expression of nuclear factor-κB and proinflammatory cytokines, and reversed the decrease in mRNA and protein expression of testosterone synthases, serum testosterone level, and sperm quality. These changes were not observed in the OHE group mice. Obesity-induced testicular oxidative stress and inflammatory response decreased testosterone synthesis and sperm quality. Moderate-load exercise alleviated the negative effect of obesity on male reproductive function by decreasing testicular oxidative stress and inflammatory responses. Although high-load exercise effectively reduced body fat, its effects on alleviating oxidative stress and improving male reproductive function were limited.
Copyright © 2020 Xuejie Yi et al.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the publication of this article.
Figures
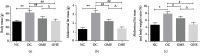
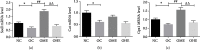
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

