Risk stratification biomarkers for Staphylococcus aureus bacteraemia
- PMID: 32082571
- PMCID: PMC7018520
- DOI: 10.1002/cti2.1110
Risk stratification biomarkers for Staphylococcus aureus bacteraemia
Abstract
Objectives: To identify risk stratification biomarkers to enrich for the subset of Staphylococcus aureus bacteraemia patients who develop deep-seated tissue infections with high morbidity and mortality to guide clinical trial enrolment and clinical management.
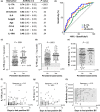
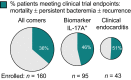
Methods: We evaluated the prognostic value of eight biomarkers for persistent bacteraemia, mortality and endovascular infection foci in a validation cohort of 160 patients with S. aureus bacteraemia enrolled consecutively over 3 years.
Results: High levels of IL-17A, IL-10 or soluble E-selectin at bacteraemia diagnosis correlated with the duration of positive blood cultures. When thresholds defined in an independent cohort were applied, these biomarkers were robust predictors of persistent bacteraemia or endovascular infection. High serum levels of IL-17A and IL-10 often preceded the radiographic diagnosis of infective endocarditis, suggesting potential utility for prioritising diagnostic radiographic imaging. High IL-8 was prognostic for all-cause mortality, while IL-17A and IL-10 were superior to clinical metrics in discriminating between attributable mortality and non-attributable mortality. High IL-17A and IL-10 identified more patients who developed microbiological failure or mortality than were identified by infective endocarditis diagnosis.
Conclusion: These biomarkers offer potential utility to identify patients at risk of persistent bacteraemia to guide diagnostic imaging and clinical management. Low biomarker levels could be used to rule out the need for more invasive TEE imaging in patients at lower risk of infective endocarditis. These biomarkers could enable clinical trials by enriching for patients with the greatest need for novel therapies.
Keywords: Staphylococcus aureus; bacteraemia; endocarditis; prognostic biomarkers.
© 2020 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology Inc.
Conflict of interest statement
YC, AOG, MCP, OM, KH, MC‐T and CMR were employees of Genentech, Inc. during the execution of this study. VGF served as Chair of V710 Scientific Advisory Committee (Merck); has received grant support from Cerexa/Actavis/Allergan, Pfizer, Advanced Liquid Logics, NIH, MedImmune, Basilea, Karius, ContraFect, Regeneron and Genentech; has NIH STTR/SBIR grants pending with Affinergy, Locus and Medical Surface, Inc; has been a paid consultant for Achaogen, Astellas, Arsanis, Affinergy, Basilea, Bayer, Cerexa, ContraFect, Cubist, Debiopharm, Durata, Grifols, Genentech, MedImmune, Merck, Medicines Co., Pfizer, Novartis, NovaDigm, Theravance and xBiotech; has received honoraria from Theravance and Green Cross; and has a patent pending in sepsis diagnostics. Other authors have no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
