Increased Risk of Liver Cirrhosis during Azathioprine Therapy for Crohn's Disease
- PMID: 32082651
- PMCID: PMC7008285
- DOI: 10.1155/2020/6726384
Increased Risk of Liver Cirrhosis during Azathioprine Therapy for Crohn's Disease
Abstract
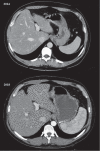
Azathioprine is a cornerstone of the therapy of Crohn's disease. Unfortunately, infections and malignancies are relatively common adverse effects related to this drug; however, cirrhosis is exceptionally reported as a side effect. We report the case of a 49-year-old male patient with ileocolonic steno-penetrating Crohn's disease who developed hepatic cirrhosis while treated with azathioprine. After taking azathioprine for 3 years with regular follow-up, he developed pancytopenia, and liver cirrhosis was diagnosed with ultrasound, abdomen computed tomography scan, transient elastography, and liver biopsy. As all other causes of liver damage were excluded, azathioprine was believed to be the cause of liver injury and therefore was interrupted.
Copyright © 2020 Jenny Roselli et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials