Understanding and Modeling Metastasis Biology to Improve Therapeutic Strategies for Combating Osteosarcoma Progression
- PMID: 32082995
- PMCID: PMC7006476
- DOI: 10.3389/fonc.2020.00013
Understanding and Modeling Metastasis Biology to Improve Therapeutic Strategies for Combating Osteosarcoma Progression
Abstract
Osteosarcoma is a malignant primary tumor of bone, arising from transformed progenitor cells with osteoblastic differentiation and osteoid production. While categorized as a rare tumor, most patients diagnosed with osteosarcoma are adolescents in their second decade of life and underscores the potential for life changing consequences in this vulnerable population. In the setting of localized disease, conventional treatment for osteosarcoma affords a cure rate approaching 70%; however, survival for patients suffering from metastatic disease remain disappointing with only 20% of individuals being alive past 5 years post-diagnosis. In patients with incurable disease, pulmonary metastases remain the leading cause for osteosarcoma-associated mortality; yet identifying new strategies for combating metastatic progression remains at a scientific and clinical impasse, with no significant advancements for the past four decades. While there is resonating clinical urgency for newer and more effective treatment options for managing osteosarcoma metastases, the discovery of druggable targets and development of innovative therapies for inhibiting metastatic progression will require a deeper and more detailed understanding of osteosarcoma metastasis biology. Toward the goal of illuminating the processes involved in cancer metastasis, a convergent science approach inclusive of diverse disciplines spanning the biology and physical science domains can offer novel and synergistic perspectives, inventive, and sophisticated model systems, and disruptive experimental approaches that can accelerate the discovery and characterization of key processes operative during metastatic progression. Through the lens of trans-disciplinary research, the field of comparative oncology is uniquely positioned to advance new discoveries in metastasis biology toward impactful clinical translation through the inclusion of pet dogs diagnosed with metastatic osteosarcoma. Given the spontaneous course of osteosarcoma development in the context of real-time tumor microenvironmental cues and immune mechanisms, pet dogs are distinctively valuable in translational modeling given their faithful recapitulation of metastatic disease progression as occurs in humans. Pet dogs can be leveraged for the exploration of novel therapies that exploit tumor cell vulnerabilities, perturb local microenvironmental cues, and amplify immunologic recognition. In this capacity, pet dogs can serve as valuable corroborative models for realizing the science and best clinical practices necessary for understanding and combating osteosarcoma metastases.
Keywords: canine cancer; comparative oncology; experimental models; metastasis biology; translational therapeutics.
Copyright © 2020 Fan, Roberts and Lizardo.
Figures


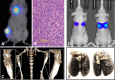
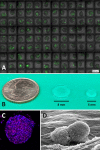
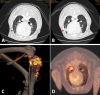
References
-
- Geller DS, Gorlick R. Osteosarcoma: a review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. (2010) 8:705–18. - PubMed
-
- Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Korholz D, et al. Long-term results of the co-operative German-Austrian-Swiss osteosarcoma study group's protocol COSS-86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Ann Oncol. (1998) 9:893–9. 10.1023/A:1008391103132 - DOI - PubMed
-
- Marina NM, Pratt CB, Rao BN, Shema SJ, Meyer WH. Improved prognosis of children with osteosarcoma metastatic to the lung(s) at the time of diagnosis. Cancer. (1992) 70:2722–7. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

