Anti-PLA2R1 Antibodies Containing Sera Induce In Vitro Cytotoxicity Mediated by Complement Activation
- PMID: 32083137
- PMCID: PMC7012209
- DOI: 10.1155/2019/1324804
Anti-PLA2R1 Antibodies Containing Sera Induce In Vitro Cytotoxicity Mediated by Complement Activation
Abstract
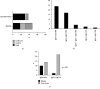
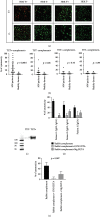
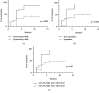
The phospholipase A2 receptor (PLA2R1) is the major autoantigen in idiopathic membranous nephropathy (MN). However, the pathogenic role of anti-PLA2R1 autoantibodies is unclear. Our aim was to evaluate the in vitro cytotoxicity of anti-PLA2R1 antibodies mediated by complement. Forty-eight patients with PLA2R1-related MN from the prospective cohort SOURIS were included. Anti-PLA2R1 titer, epitope profile, and anti-PLA2R1 IgG subclasses were characterized by ELISA. Cell cytotoxicity was evaluated by immunofluorescence in HEK293 cells overexpressing PLA2R1 incubated with patient or healthy donor sera in the presence or absence of rabbit complement or complement inhibitors. Mean cytotoxicity of anti-PLA2R1 sera for HEK293 cells overexpressing PLA2R1 was 2 ± 2%, which increased to 24 ± 6% after addition of rabbit complement (p < 0.001) (n = 48). GVB-EDTA, which inhibits all complement activation pathways, completely blocked cell cytotoxicity, whereas Mg-EGTA, which only inhibits the classical and lectin pathways, highly decreased suggesting a limited role of the alternative pathway. A higher diversity of IgG subclasses beyond IgG4 and high titer of total IgG anti-PLA2R1 were associated with increased cytotoxicity (p = 0.01 and p = 0.03 respectively). In a cohort of 37 patients treated with rituximab, high level of complement-mediated cytotoxicity was associated with less and delayed remission at month 6 after rituximab therapy (5/12 vs. 20/25 (p = 0.03) in 8.5 months ± 4.4 vs. 4.8 ± 4.0 (p = 0.02)). Kaplan-Meier analysis demonstrated that high level of cytotoxicity (≥40%) (p = 0.005), epitope spreading (defined by immunization beyond the immunodominant CysR domain) (p = 0.002), and high titer of anti-PLA2R1 total IgG (p = 0.01) were factors of poor renal prognosis. Anti-PLA2R1 antibodies containing sera can induce in vitro cytotoxicity mediated by complement activation, and the level of cytotoxicity increases with the diversity and the titer of anti-PLA2R1 IgG subclasses. These patients with high level of complement-mediated cytotoxicity could benefit from adjuvant therapy using complement inhibitor associated with rituximab to induce earlier remission and less podocyte injury.
Copyright © 2019 Maël Lateb et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

