A High-Throughput Method for Characterizing Novel Chimeric Antigen Receptors in Jurkat Cells
- PMID: 32083149
- PMCID: PMC7021643
- DOI: 10.1016/j.omtm.2020.01.012
A High-Throughput Method for Characterizing Novel Chimeric Antigen Receptors in Jurkat Cells
Abstract
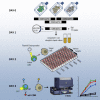
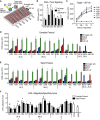
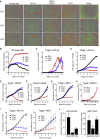
Chimeric antigen receptor (CAR) development involves extensive empirical characterization of antigen-binding domain (ABD)/CAR constructs for clinical suitability. Here, we present a cost-efficient and rapid method for evaluating CARs in human Jurkat T cells. Using a modular CAR plasmid, a highly efficient ABD cloning strategy, plasmid electroporation, short-term co-culture, and flow-cytometric detection of CD69, this assay (referred to as CAR-J) evaluates sensitivity and specificity for ABDs. Assessing 16 novel anti-CD22 single-chain variable fragments derived from mouse monoclonal antibodies, CAR-J stratified constructs by response magnitude to CD22-expressing target cells. We also characterized 5 novel anti-EGFRvIII CARs for preclinical development, identifying candidates with varying tonic and target-specific activation characteristics. When evaluated in primary human T cells, tonic/auto-activating (without target cells) EGFRvIII-CARs induced target-independent proliferation, differentiation toward an effector phenotype, elevated activity against EGFRvIII-negative cells, and progressive loss of target-specific response upon in vitro re-challenge. These EGFRvIII CAR-T cells also showed anti-tumor activity in xenografted mice. In summary, CAR-J represents a straightforward method for high-throughput assessment of CAR constructs as genuine cell-associated antigen receptors that is particularly useful for generating large specificity datasets as well as potential downstream CAR optimization.
Keywords: CAR-T; CD69; EGFRvIII; Jurkat; T cell; high-throughput; live imaging; plasmid; pre-clinical; screening.
Crown Copyright © 2020.
Figures





References
-
- Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P.T., Carpenter R.O., Stetler-Stevenson M., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J. Clin. Oncol. 2015;33:540–549. - PMC - PubMed
-
- Grigor E.J.M., Fergusson D.A., Haggar F., Kekre N., Atkins H., Shorr R., Holt R.A., Hutton B., Ramsay T., Seftel M. Efficacy and safety of chimeric antigen receptor T-cell (CAR-T) therapy in patients with haematological and solid malignancies: protocol for a systematic review and meta-analysis. BMJ Open. 2017;7:e019321. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

