Transcatheter treatment of native aortic valve regurgitation: Results from an international registry using the transfemoral ACURATE neo valve
- PMID: 32083165
- PMCID: PMC7016455
- DOI: 10.1016/j.ijcha.2020.100480
Transcatheter treatment of native aortic valve regurgitation: Results from an international registry using the transfemoral ACURATE neo valve
Abstract
Background: Transcatheter aortic valve replacement (TAVR) has been validated for the treatment of severe symptomatic aortic stenosis in patients at high and intermediate surgical risk. Recently, TAVR has been proposed as an alternative to medical therapy in inoperable patients with severe native aortic valve regurgitation (NAVR). This multicenter international registry sought to evaluate safety and efficacy of TAVR with the self-expandable ACURATE neo valve in a cohort of patients with NAVR.
Methods: A total of 24 patients with severe NAVR treated by TAVR between September 2016 and October 2018 in 13 European centers were included. Clinical, procedural and follow up data were inserted in a dedicated database. Outcomes were codified according to Valve Academic Research Consortium-2 criteria.
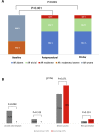
Results: Mean age was 79.4 years, 58.4% were female. Mean EuroSCORE II and STS score were 5% and 3.9%, respectively. Device success was 87.5%. Moderate paravalvular leak (PVL) was found in two (8.3%) of patients, both with a perimeter oversizing index <10%. Implantation of a second device was necessary in three cases (12.5%), one for severe PVL and two for device displacement. New pacemaker implantation rate was 21.1%. At 30 days, stroke and all-cause mortality rates were 0% and 4.1%, respectively.
Conclusions: This multicenter study suggests good feasibility and early safety of transfemoral TAVR with the self-expandable ACURATE neo device in patients with severe NAVR refused for surgery. Rates of moderate PVL, new pacemaker implantation and need for a second valve were higher than those reported for TAVR in aortic stenosis.
Keywords: AR, aortic regurgitation; AS, aortic stenosis; Acurate Neo; CHF, congestive heart failure; NAVR, native aortic valve regurgitation; NYHA, New York Heart Association; Native aortic valve regurgitation; PVL, paravalvular leak; TAVR, transcatheter aortic valve replacement; THV, transcatheter heart valve; Transcatheter aortic valve replacement; VARC 2, Valve Academic Research Consortium 2.
© 2020 The Authors.
Conflict of interest statement
Ole de Backer: consultant for Abbott; Won-Keun Kim: received proctor fees from Symetis SA/Boston Scientific, St. Jude Medical/Abbott, lecture honoraria from Edwards Lifesciences, Symetis SA/Boston, St. Jude Medical/Abbott; Luis Nombela-Franco received proctor fees from Abbott, lecture honoraria from Edwards Lifesciences and Boston Scientific; Moritz Seiffert received lecture honorary or congress travel support from Abbott, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic and Symetis. Fausto Castriota received proctor fes from Boston Scientific. Dr. Bedogni has served as a consultant for Abbott Vascular, Medtronic, Boston Scientific, and Terumo. Lars Sondergaard: received research grants and has been consultant for Abbott, Medtronic and Boston Scientific. Giuseppe Tarantini: proctor for Boston Scientific and received lecture fees from Edwards Lifesciences and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

References
-
- Baumgartner H., Falk V., Bax J.J., De Bonis M., Hamm C., Holm P.J. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017;38:2739–2791. - PubMed
-
- Mack M.J., Leon M.B., Thourani V.H., Makkar R., Kodali S.K., Russo M. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019;380:1695–1705. - PubMed
-
- Ueshima D., Fovino L.N., D'Amico G., Brener S.J., Esposito G., Tarantini G. Transcatheter versus surgical aortic valve replacement in low- and intermediate-risk patients: an updated systematic review and meta-analysis. Cardiovasc. Interv. Ther. 2019;34:216–225. - PubMed
-
- Tarantini G., Nai Fovino L., D'Errigo P., Rosato S., Barbanti M., Tamburino C. Factors influencing the choice between transcatheter and surgical treatment of severe aortic stenosis in patients younger than 80 years: results from the OBSERVANT study. Catheter. Cardiovasc. Interv. 2019 - PubMed
-
- Cribier A. Commemorating the 15-year anniversary of TAVI: insights into the early stages of development, from concept to human application, and perspectives. EuroIntervention. 2017;13:29–37. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

