Down-regulation of microRNA-203a suppresses IL-1β-induced inflammation and cartilage degradation in human chondrocytes through Smad3 signaling
- PMID: 32083281
- PMCID: PMC7070148
- DOI: 10.1042/BSR20192723
Down-regulation of microRNA-203a suppresses IL-1β-induced inflammation and cartilage degradation in human chondrocytes through Smad3 signaling
Abstract
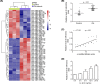
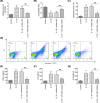
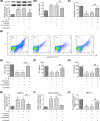
Osteoarthritis (OA) is a chronic and prevalent degenerative musculoskeletal disorder, which is characterized by articular cartilage degradation and joint inflammation. MicroRNA-203a (miR-203a) has been shown to be involved in multiple pathological processes during OA, but little is known about its function in chondrocyte extracellular matrix (ECM) degradation. In the present study, we aimed to elucidate the effects of miR-203a on articular cartilage degradation and joint inflammation. We observed that the miR-203a level was significantly up-regulated in OA tissues and in an in vitro model of OA, respectively. Inhibition of miR-203a significantly alleviated the interleukin (IL)-1β-induced inflammatory response and ECM degradation in chondrocytes. Moreover, mothers against decapentaplegic homolog 3 (Smad3), a key factor in maintaining chondrocyte homeostasis, was identified as a putative target of miR-203a in chondrocytes. More importantly, inhibition of Smad3 impaired the inhibitory effects of the miR-203a on IL-1β-induced inflammatory response and ECM degradation. Collectively, these results demonstrated that miR-203a may contribute to articular cartilage degradation of OA by targeting Smad3, suggesting a novel therapeutic target for the treatment of OA.
Keywords: ECM; Osteoarthritis; articular cartilage; inflammatory cytokines; miR-203a.
© 2020 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures


References
-
- Swingler T.E. et al. . (2011) The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum. 64, 1909–1919 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

