A memory switch for plant synthetic biology based on the phage ϕC31 integration system
- PMID: 32083668
- PMCID: PMC7102980
- DOI: 10.1093/nar/gkaa104
A memory switch for plant synthetic biology based on the phage ϕC31 integration system
Abstract
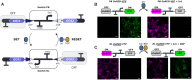
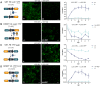
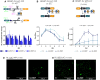
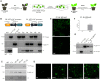
Synthetic biology has advanced from the setup of basic genetic devices to the design of increasingly complex gene circuits to provide organisms with new functions. While many bacterial, fungal and mammalian unicellular chassis have been extensively engineered, this progress has been delayed in plants due to the lack of reliable DNA parts and devices that enable precise control over these new synthetic functions. In particular, memory switches based on DNA site-specific recombination have been the tool of choice to build long-term and stable synthetic memory in other organisms, because they enable a shift between two alternative states registering the information at the DNA level. Here we report a memory switch for whole plants based on the bacteriophage ϕC31 site-specific integrase. The switch was built as a modular device made of standard DNA parts, designed to control the transcriptional state (on or off) of two genes of interest by alternative inversion of a central DNA regulatory element. The state of the switch can be externally operated by action of the ϕC31 integrase (Int), and its recombination directionality factor (RDF). The kinetics, memory, and reversibility of the switch were extensively characterized in Nicotiana benthamiana plants.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Liu W., Neal Stewart C.. Plant synthetic biology. Trends Plant Sci. 2015; 20:309–317. - PubMed
-
- Baltes N.J., Voytas D.F.. Enabling plant synthetic biology through genome engineering. Trends Biotechnol. 2015; 33:120–131. - PubMed
-
- Ye X. Engineering the provitamin A (-Carotene) biosynthetic pathway into (Carotenoid-Free) rice endosperm. Science. 2000; 287:303–305. - PubMed
-
- Rogers C., Oldroyd G.E.D.. Synthetic biology approaches to engineering the nitrogen symbiosis in cereals. J. Exp. Bot. 2014; 65:1939–1946. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

