Transposon mutagenesis in Mycobacterium kansasii links a small RNA gene to colony morphology and biofilm formation and identifies 9,885 intragenic insertions that do not compromise colony outgrowth
- PMID: 32083796
- PMCID: PMC7142372
- DOI: 10.1002/mbo3.988
Transposon mutagenesis in Mycobacterium kansasii links a small RNA gene to colony morphology and biofilm formation and identifies 9,885 intragenic insertions that do not compromise colony outgrowth
Abstract
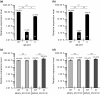
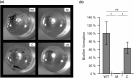
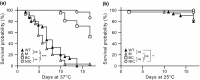
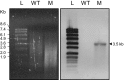
Mycobacterium kansasii (Mk) is a resilient opportunistic human pathogen that causes tuberculosis-like chronic pulmonary disease and mortality stemming from comorbidities and treatment failure. The standard treatment of Mk infections requires costly, long-term, multidrug courses with adverse side effects. The emergence of drug-resistant isolates further complicates the already challenging drug therapy regimens and threatens to compromise the future control of Mk infections. Despite the increasingly recognized global burden of Mk infections, the biology of this opportunistic pathogen remains essentially unexplored. In particular, studies reporting gene function or generation of defined mutants are scarce. Moreover, no transposon (Tn) mutagenesis tool has been validated for use in Mk, a situation limiting the repertoire of genetic approaches available to accelerate the dissection of gene function and the generation of gene knockout mutants in this poorly characterized pathogen. In this study, we validated the functionality of a powerful Tn mutagenesis tool in Mk and used this tool in conjunction with a forward genetic screen to establish a previously unrecognized role of a conserved mycobacterial small RNA gene of unknown function in colony morphology features and biofilm formation. We also combined Tn mutagenesis with next-generation sequencing to identify 12,071 Tn insertions that do not compromise viability in vitro. Finally, we demonstrated the susceptibility of the Galleria mellonella larva to Mk, setting the stage for further exploration of this simple and economical infection model system to the study of this pathogen.
Keywords: Galleria mellonella; Mycobacterium kansasii; biofilm; gene essentiality; nontuberculous mycobacteria; small noncoding RNA.
© 2020 The Authors. MicrobiologyOpen published by John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures
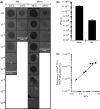
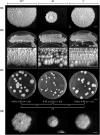
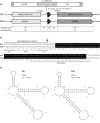
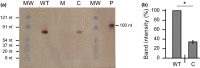




References
-
- Bardarov, S. , Kriakov, J. , Carriere, C. , Yu, S. , Vaamonde, C. , McAdam, R. A. , … Jacobs, W. R. (1997). Conditionally replicating mycobacteriophages: A system for transposon delivery to Mycobacterium tuberculosis . Proceedings of the National Academy of Sciences of the United States of America, 94(20), 10961–10966. 10.1073/pnas.94.20.10961 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

