Stab2-Mediated Clearance of Supramolecular Polymer Nanoparticles in Zebrafish Embryos
- PMID: 32083854
- PMCID: PMC9665412
- DOI: 10.1021/acs.biomac.9b01318
Stab2-Mediated Clearance of Supramolecular Polymer Nanoparticles in Zebrafish Embryos
Abstract
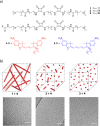
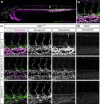
Supramolecular polymers are attractive scaffolds for use as nanocarriers in drug delivery thanks to their modularity and easy fabrication; however, a molecular view into their in vivo behavior is lacking. Herein, we prepare fluorescent squaramide-based supramolecular polymer nanoparticles that range from fibers to spheres while maintaining their surface chemistry and near-neutral surface charge by a co-assembly approach involving a sulfo-cyanine-labeled monomer to track their in vivo biodistribution behavior and clearance in optically transparent zebrafish embryos. Evasion of macrophages, localization of the fibrillar aggregates in the caudal vein, and association with scavenger endothelial cells are observed. The interaction of the fibrillar supramolecular nanoparticles with the caudal vein is abrogated in gene-edited zebrafish lacking Stabilin-2, a receptor analogously found in the mammalian liver, providing a molecular view into their interaction with scavenger endothelial cells. We further show that this interaction can be tuned based on the choice of monomer and its resultant self-assembly.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Directing Nanoparticle Biodistribution through Evasion and Exploitation of Stab2-Dependent Nanoparticle Uptake.ACS Nano. 2018 Mar 27;12(3):2138-2150. doi: 10.1021/acsnano.7b06995. Epub 2018 Jan 18. ACS Nano. 2018. PMID: 29320626 Free PMC article.
-
Stabilin 1 and 2 are important regulators for cellular uptake of apolipoprotein B-containing lipoproteins in zebrafish.Atherosclerosis. 2022 Apr;346:18-25. doi: 10.1016/j.atherosclerosis.2022.02.018. Epub 2022 Feb 28. Atherosclerosis. 2022. PMID: 35247629
-
Stabilin-1 is required for the endothelial clearance of small anionic nanoparticles.Nanomedicine. 2021 Jun;34:102395. doi: 10.1016/j.nano.2021.102395. Epub 2021 Apr 8. Nanomedicine. 2021. PMID: 33838334
-
Supramolecular structures and self-association processes in polymer systems.Physiol Res. 2016 Oct 20;65(Suppl 2):S165-S178. doi: 10.33549/physiolres.933419. Physiol Res. 2016. PMID: 27762583 Review.
-
Supramolecular Nanomedicine - An Overview.Curr Drug Targets. 2015;16(13):1407-28. doi: 10.2174/1389450115666140804223539. Curr Drug Targets. 2015. PMID: 25090984 Review.
Cited by
-
Zebrafish Models for the Safety and Therapeutic Testing of Nanoparticles with a Focus on Macrophages.Nanomaterials (Basel). 2021 Jul 9;11(7):1784. doi: 10.3390/nano11071784. Nanomaterials (Basel). 2021. PMID: 34361170 Free PMC article. Review.
-
Zebrafish as a platform to evaluate the potential of lipidic nanoemulsions for gene therapy in cancer.Front Pharmacol. 2022 Oct 31;13:1007018. doi: 10.3389/fphar.2022.1007018. eCollection 2022. Front Pharmacol. 2022. PMID: 36386231 Free PMC article.
-
Role of the Hyaluronan Receptor, Stabilin-2/HARE, in Health and Disease.Int J Mol Sci. 2020 May 15;21(10):3504. doi: 10.3390/ijms21103504. Int J Mol Sci. 2020. PMID: 32429122 Free PMC article. Review.
References
-
- Wilhelm S.; Tavares A. J.; Dai Q.; Ohta S.; Audet J.; Dvorak H. F.; Chan W. C. W. Analysis of Nanoparticle Delivery to Tumours. Nat. Rev. Mater. 2016, 1, 1601410.1038/natrevmats.2016.14. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

