CART-WHEEL.org: An Ethically Approved Online Database for Patient-Entered Data to Facilitate Rare Cancer Research
- PMID: 32083956
- PMCID: PMC7049250
- DOI: 10.1200/CCI.19.00085
CART-WHEEL.org: An Ethically Approved Online Database for Patient-Entered Data to Facilitate Rare Cancer Research
Abstract
Purpose: Rare cancers are challenging for researchers, as clinicians and scientists have difficulty recruiting sufficient patient cases to power studies appropriately. Likewise, patients often are frustrated by a lack of specific information or evidence base for their cancer and, although eager to participate in research, have limited opportunities. We established CART-WHEEL.org, an online patient-entered database, to directly engage patients in the research process, collect rare cancer data, and facilitate their entry into additional research.
Patients and methods: Patients access CART-WHEEL.org directly online. Clinical information is collected from users via a streamlined questionnaire developed collaboratively with consumer groups to ensure accessibility and relevance. Data collected include the following: patient demographics, comorbidities, and risk factors and tumor diagnostic, biomarker, and treatment history. Patients can download a medical summary for personal use; consent for research use of data; and indicate willingness to be contacted about other research or clinical trials. We describe data collected to date and its validation, and we provide examples of how CART-WHEEL.org can facilitate rare cancer research.
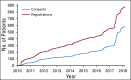
Results: From January 2010 to March 2018, 558 patients provided consent and entered their rare cancer data. One hundred distinct rare tumor types and patients from 22 countries were included. Validation of data entered by 21 patients with sarcoma against a hospital database demonstrated accuracy sufficient to facilitate future research in key fields, such as tumor site (95%) and histopathologic diagnosis (90%). Examples of CART-WHEEL-based disease-specific projects, subsequent recruitment to other rare cancer projects, and rare cancer patient cases of interest are described.
Conclusions: Online platforms like CART-WHEEL.org can engage consumers directly, facilitating collection of patient-entered rare cancer data for hypothesis generation, and connect patients with researchers to enable specific rare cancer research and clinical trials.
Conflict of interest statement
Katherine M. Tucker
Michael Friedlander
Peter Gibbs
Jayesh Desai
Judith Trotman
Clare L. Scott
No other potential conflicts of interest were reported.
Figures


References
-
- Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: The rare cancer burden in Europe. Eur J Cancer. 2011;47:2493–2511. - PubMed
-
- Gaddipati H, Liu K, Pariser A, et al. Rare cancer trial design: Lessons from FDA approvals. Clin Cancer Res. 2012;18:5172–5178. - PubMed
-
- Dockser Marcus A. To make progress in rare cancers, patients must lead the way. J Clin Oncol. 2009;27:2575–2577. - PubMed
-
- Blay J-Y, Coindre J-M, Ducimetière F, et al. The value of research collaborations and consortia in rare cancers. Lancet Oncol. 2016;17:e62–e69. - PubMed
-
- Nunn JS, Scott CL, Stubbs, JW, Cherry SF: Involving the public in rare cancer care and research: shaping the future for rare and uncommon cancers, in Raghavan D, Ahluwalia MS, Blanke CD, et al (eds): Textbook of Uncommon Cancer (ed 5). West Sussex, United Kingdom, Wiley-Blackwell, 2017, pp12-18.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

