Single-cell glycolytic activity regulates membrane tension and HIV-1 fusion
- PMID: 32084246
- PMCID: PMC7055913
- DOI: 10.1371/journal.ppat.1008359
Single-cell glycolytic activity regulates membrane tension and HIV-1 fusion
Erratum in
-
Correction: Single-cell glycolytic activity regulates membrane tension and HIV-1 fusion.PLoS Pathog. 2021 May 10;17(5):e1009584. doi: 10.1371/journal.ppat.1009584. eCollection 2021 May. PLoS Pathog. 2021. PMID: 33970974 Free PMC article.
Abstract
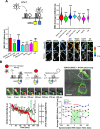
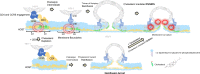
There has been resurgence in determining the role of host metabolism in viral infection yet deciphering how the metabolic state of single cells affects viral entry and fusion remains unknown. Here, we have developed a novel assay multiplexing genetically-encoded biosensors with single virus tracking (SVT) to evaluate the influence of global metabolic processes on the success rate of virus entry in single cells. We found that cells with a lower ATP:ADP ratio prior to virus addition were less permissive to virus fusion and infection. These results indicated a relationship between host metabolic state and the likelihood for virus-cell fusion to occur. SVT revealed that HIV-1 virions were arrested at hemifusion in glycolytically-inactive cells. Interestingly, cells acutely treated with glycolysis inhibitor 2-deoxyglucose (2-DG) become resistant to virus infection and also display less surface membrane cholesterol. Addition of cholesterol in these in glycolytically-inactive cells rescued the virus entry block at hemifusion and enabled completion of HIV-1 fusion. Further investigation with FRET-based membrane tension and membrane order reporters revealed a link between host cell glycolytic activity and host membrane order and tension. Indeed, cells treated with 2-DG possessed lower plasma membrane lipid order and higher tension values, respectively. Our novel imaging approach that combines lifetime imaging (FLIM) and SVT revealed not only changes in plasma membrane tension at the point of viral fusion, but also that HIV is less likely to enter cells at areas of higher membrane tension. We therefore have identified a connection between host cell glycolytic activity and membrane tension that influences HIV-1 fusion in real-time at the single-virus fusion level in live cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

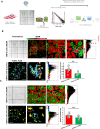
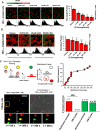

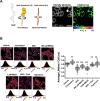
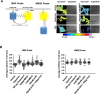
References
-
- Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 Entry Cofactor: Functional cDNA Cloning of a Seven-Transmembrane, G Protein-Coupled Receptor. Science (80-). 1996;272(May). - PubMed
-
- Gallo SA, Finnegan CM, Viard M, Raviv Y, Dimitrov A, Rawat SS, et al. The HIV Env-mediated fusion reaction. Biochim Biophys Acta—Biomembr. 2003;1614:36–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

