A Calreticulin Tail: C-terminal Mutants of Calreticulin Allow Cancer Cells to Evade Phagocytosis
- PMID: 32084350
- PMCID: PMC7594649
- DOI: 10.1016/j.molcel.2020.01.024
A Calreticulin Tail: C-terminal Mutants of Calreticulin Allow Cancer Cells to Evade Phagocytosis
Abstract
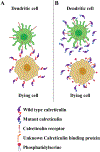
In the current issue of Molecular Cell, Liu et al. (2020) show that the secretion of cancer-linked forms of mutant calreticulin allow cancer cells to escape protective immune responses induced by chemotherapeutic and immunotherapeutic drugs, thereby promoting tumor growth.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures
Comment on
-
Immunosuppression by Mutated Calreticulin Released from Malignant Cells.Mol Cell. 2020 Feb 20;77(4):748-760.e9. doi: 10.1016/j.molcel.2019.11.004. Epub 2019 Nov 27. Mol Cell. 2020. PMID: 31785928
References
-
- Boncompain G, Divoux S, Gareil N, De Forges H, Lescure A, Latreche L, Mercanti V, Jollivet F, Raposo G, Perez F, 2012. Nat. Methods 9, 493–498. - PubMed
-
- Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G, 2015. Cancer Cell 28, 690–714. - PubMed
-
- Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, Bratton DL, Oldenborg PA, Michalak M, Henson PM, 2005. Cell 123, 321–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

